Impact of body mass index on stromal vascular fraction cell yield from human lipoaspirates
- Regenerative Medicine Unit, Lucknow, Uttar Pradesh, India
Abstract
Background: The stromal vascular fraction (SVF) of adipose tissue is a critical source of regenerative cells for autologous therapies. While donor-related factors like age have been studied, the influence of Body Mass Index (BMI) on SVF yield remains unclear. This study aimed to determine if a correlation exists between patient BMI and the number of nucleated cells isolated from lipoaspirates.
Methods: Adipose tissue was collected from 48 female donors undergoing elective liposuction. Samples were processed using a standardized, point-of-care system (GID platform) for SVF isolation. The total nucleated cell count per milliliter of processed adipose tissue was determined and correlated with the donor's BMI.
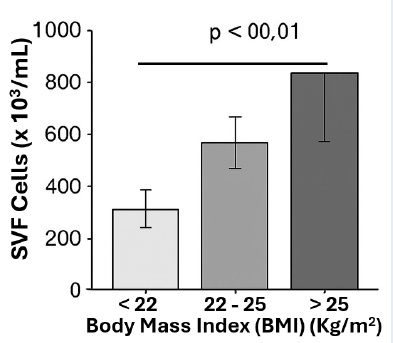
Results: The mean SVF yield was cells/mL. A significant positive correlation (Pearson r = 0.52, P < 0.001) was observed between increasing BMI and SVF cell yield. Donors with a BMI ≥ 25 kg/m² yielded significantly more cells than those with a BMI < 22 kg/m².
Conclusions: Using a consistent harvesting site and processing technique, we conclude that higher BMI in female patients is associated with a greater yield of SVF cells from abdominal adipose tissue. This suggests that adipose tissue cellularity or composition varies with body mass, which has implications for pre-surgical planning in cell-based therapies.
Introduction
The use of the stromal vascular fraction (SVF) in regenerative medicine continues to expand due to its rich content of mesenchymal stromal cells and progenitor cells 1,2. The efficiency of these therapies often depends on the number of viable cells obtained, making the optimization of SVF yield a primary concern 3.
Multiple factors can influence cell yield, including the harvesting technique, anatomic donor site, and the technology used for processing 4,5. Donor-specific biological variables, particularly age, have been investigated with conflicting results 6,7. Another key patient characteristic is Body Mass Index (BMI), which reflects overall adiposity. It is plausible that the biological composition of adipose tissue—including its vascularity, cellular density, and extracellular matrix—differs between individuals of normal weight and those who are overweight 8.
Some studies suggest that adipose tissue in individuals with higher BMI may be characterized by hypertrophy and a pro-inflammatory state 9, but its effect on the yield of stromal cells after enzymatic digestion is not well-defined. Contradictory reports exist, with some indicating no correlation 10 and others suggesting a potential increase in progenitor cells 11.
During our clinical experience, we observed notable variations in SVF yield that appeared to be independent of age or processing variables. We therefore hypothesized that a patient's BMI significantly influences the SVF cell yield obtained from processed lipoaspirates.
Methods
Patients
This study included 48 healthy female donors scheduled for cosmetic abdominal liposuction and autologous fat grafting. All participants provided informed consent, and the study was approved by the local ethics committee. To control for variables, all adipose tissue samples were harvested from the subcutaneous abdominal depot. Donors with metabolic disorders or on medications known to affect adipose tissue biology were excluded. Patient demographics are summarized in
Patient Demographics (n=48)
| Characteristic | Value (Mean ± SD or Range) |
|---|---|
| Sex | All Female |
| Age (years) | 42.5 ± 12.8 |
| BMI (kg/m2) | 24.1 ± 3.5 |
| Raw Lipoaspirate Volume | 1.8 - 3.2 L |
| Processed Adipose Volume | 150 - 350 mL |
Adipose Tissue Harvesting and SVF Isolation
Liposuction was performed under general anesthesia using a standard tumescent technique (Lidocaine 1%, Epinephrine 1:1000 in Lactated Ringer's solution). A power-assisted system with a 4-mm blunt cannula was used for harvesting.
SVF cells were isolated by collagenase digestion method. Briefly, the tissue was washed extensively with PBS solution to remove blood components and free lipid. The washed adipose tissue was weighed, and an equal volume of collagense solution (0.1% collagenase type II, Sigma-Aldrich, St Loiu, MO) was added for digestion. The mixute of adipose tissue and enzyme were incubated at 37 37°C, for 40 min, before it was used to centrifuge at 800g for 10 min to collect SVF cells at the bottom of the tubes. The cell pellets were wash twice with PBS before they were used for further analysis.
Cell Counting and Quality Control
Total nucleated cell count and viability were determined using the NucleoCounter NC-3000 system (ChemoMetec), which discriminates between viable and non-viable nucleated cells. Cell suspensions were also assessed for endotoxin levels (Endosafe-PTS, Charles River) and microbial sterility (72-hour culture on agar-chocolate plates). Cell viability analysis was performed by flow cytometry.
Statistical Analysis
SVF yield data (cells/mL of adipose tissue) are presented as mean ± SEM. Correlation between BMI and cell yield was analyzed using Pearson's correlation coefficient. Inter-group comparisons were performed using one-way ANOVA with a post-hoc Tukey test. A p-value of < 0.05 was considered statistically significant. All analyses were conducted with GraphPad Prism v5.1.
Results
Processing of abdominal lipoaspirates (150–350 mL adipose) consistently yielded a high-quality SVF cell suspension within 60-70 minutes. The average cell viability was 85.2% ± 7.1%.
A significant positive correlation was found between donor BMI and SVF cell yield (Pearson r = 0.52, P < 0.001) (
Correlation Analysis: SVF Yield vs. BMI
| Parameter | Value |
|---|---|
| No. of XY Pairs | 48 |
| Pearson r | 0.52 |
| 95% CI | 0.28 to 0.70 |
| P value | < 0.001 |
| R2 | 0.27 |
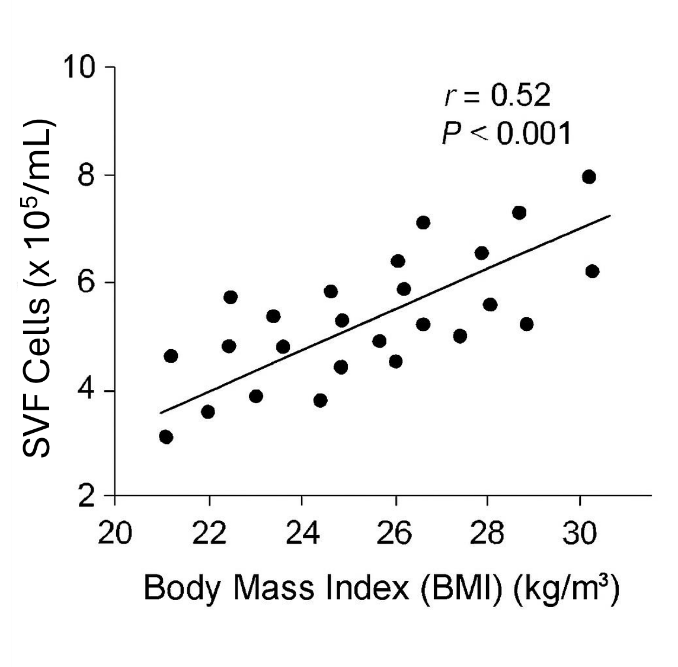
Scatter plot showing positive linear correlation between BMI (x-axis) and SVF yield (y-axis).
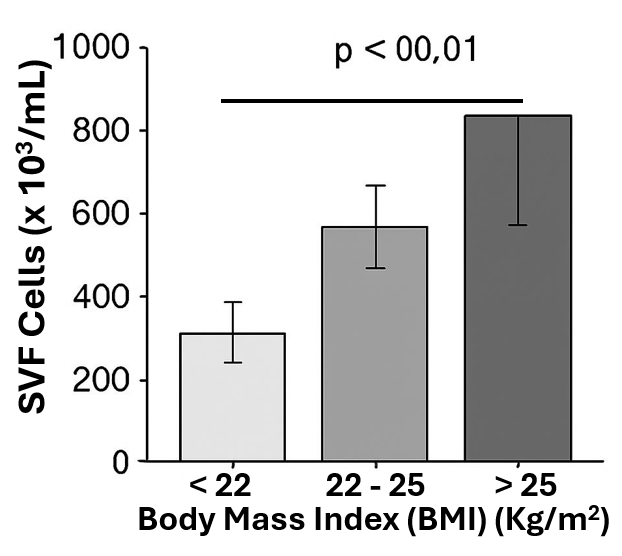
Bar graph comparing SVF yield between the lowest (<22) and highest (>25) BMI groups, showing statistical significance ( ** P < 0.01).
All final cell products were sterile, with low endotoxin levels and a high percentage (>95%) of cells in a non-proliferating, resting state (
Cell Product Characteristics (Mean ± SD)
| Parameter | Value |
|---|---|
| Nucleated Viable Cells/mL | 6.85 × 105 ± 1.92 × 105 |
| Cell Viability (%) | 85.2 ± 7.1 |
| Apoptotic Index (%) | 4.1 ± 2.0 |
| Resting Cells (%) | 95.5 ± 1.8 |
| Endotoxin (EU/mL) | 1.25 ± 0.98 |
| Microbial CFU | 0 |
Discussion
This study demonstrates a significant positive correlation between patient BMI and the yield of nucleated cells from the stromal vascular fraction of abdominal adipose tissue. Our findings indicate that, for a given volume of processed tissue, more cells can be obtained from donors with a higher BMI.
This result can be interpreted through the lens of adipose tissue biology. Higher BMI is often associated with adipose tissue hyperplasia (an increase in adipocyte number) and potentially greater vascularization to support the expanding tissue mass 12. Since SVF cells are primarily located in the perivascular niche 13, a more vascularized tissue could logically yield a higher number of these cells upon digestion. Furthermore, the endocrine and metabolic profile of adipose tissue in individuals with higher BMI, while often dysregulated, might support a larger reservoir of stromal and progenitor cells 11.
Our findings contrast with some previous reports 10, which may be due to differences in patient population, harvesting site, or the SVF processing methodology. Our use of a standardized, closed-system device with efficient washing may provide a more accurate reflection of the true stromal cell population, minimizing the confounding effect of blood-derived cells.
A critical consideration for clinical translation is the dosage of cells required for therapeutic effect. Our data suggest that for patients with a lower BMI, a larger volume of adipose tissue may need to be processed to achieve a target cell number compared to patients with a higher BMI. This has direct implications for surgical planning and patient selection for autologous cell therapies.
Conclusion
In conclusion, our data establish BMI as an important donor-specific factor influencing SVF cell yield. Further research is needed to elucidate the underlying biological mechanisms and to assess whether the functional quality of cells differs with BMI.
Abbreviations
AI: Artificial Intelligence; ANOVA: Analysis of Variance; BMI: Body Mass Index; CFU: Colony-Forming Units; PBS: Phosphate-Buffered Saline; SEM: Standard Error of the Mean; SVF: Stromal Vascular Fraction
Acknowledgments
None.
Author’s contributions
All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have used generative AI and/or AI-assisted technologies in the writing process before submission, but only to improve the language and readability of their paper.
Competing interests
The authors declare that they have no competing interests.

