Abstract
Mesenchymal stem cells (MSCs) now are popular stem cells for clinical applications. To date, MSCs were accepted in various disease treatments with several FDA approved treatments in some countries. One important requirement for the clinical usage of stem cells is the production of stem cells. Actually, the treatment efficacy of MSC transplantation depends on the quality of transplanted MSCs. This review aimed to present some guidelines for MSC production according to good manufacturing practice that helps to maintain the quality of stem cells from batch to batch as well as the clinical satisfaction.
Introduction
Stem cell transplantation is a novel treatment method for many diseases, especially degenerative diseases. There are reports of the clinical application of stem cells for more than 70 diseases. Mesenchymal stem cells (MSCs) have become popular for disease treatment in recent years via two approaches: personalized medicine (autologous transplantation) and as a stem cell drug (allogeneic transplantation) Larsen and Lewis, 2011 Squillaro et al., 2015 .
According to clinicaltrials.gov (2015), there are currently about 560 clinical trials using MSCs from several sources ( Figure 1 ). Many clinical trials are being performed in East Asia (176/560) and North America (111/560) ( Table 1 ). Although there are more than 20 diseases that can be treated by MSC transplantation, researchers have focused on two groups of diseases: degenerative and immune system-related diseases. Unlike other kinds of stem cells, MSCs exhibit two therapeutic properties including a differentiation potential for specific cell types such as osteoblasts Montespan et al., 2014 Shao et al., 2015 , chondroblasts Berninger et al., 2013 Perdisa et al., 2015 , and adipocytes Gruia et al., 2015 Lee et al., 2015 , and immunomodulation of certain kinds of immune cells such as T cells, B cells, natural killer cells, dendritic cells, and T regulatory cells Cardoso et al., 2012 Melief et al., 2013 Saeidi et al., 2013 . Therefore, in earlier clinical studies, MSCs have been differentiated into specific cells to recover the degenerated cells in injured tissues, whereas recent clinical studies have used the immunomodulation of MSCs to treat immune dysfunction.
Figure 1. Clinical trials using MSCs according to clinicaltrials.gov
Table 1. Distribution of clinical trials using MSCs worldwide
(according to clinicaltrial.gov, November 20th, 2015)
Recent studies have shown that allogeneic MSCs can perform better immunomodulation than autologous MSCs. These results triggered the use of allogeneic MSCs in clinical applications. Commercialized MSC-based products have been developed and approved as stem cell drugs in some countries ( Figure 2 ). Osteocel (NuVasive), Trinity (Orthofix), and LiquidGen (Skye Orthobiologics) use allogeneic MSCs as the main component for bone regeneration and reduction of inflammation. MSC-based products have also been approved in Canada and Korea for certain diseases. Cartistem is stem cell drug containing umbilical cord blood-derived MSCs, which was approved in Korea as a drug for osteoarthritis. In 2012, Prochymal (Osiris Therapeutics), an allogeneic MSC-based product, was approved in Canada for graft-versus-host disease treatment. To date (2015), there are nine commercialized MSC-based products approved worldwide. Interestingly, most of them are allogeneic MSC-based products ( Table 2 ).
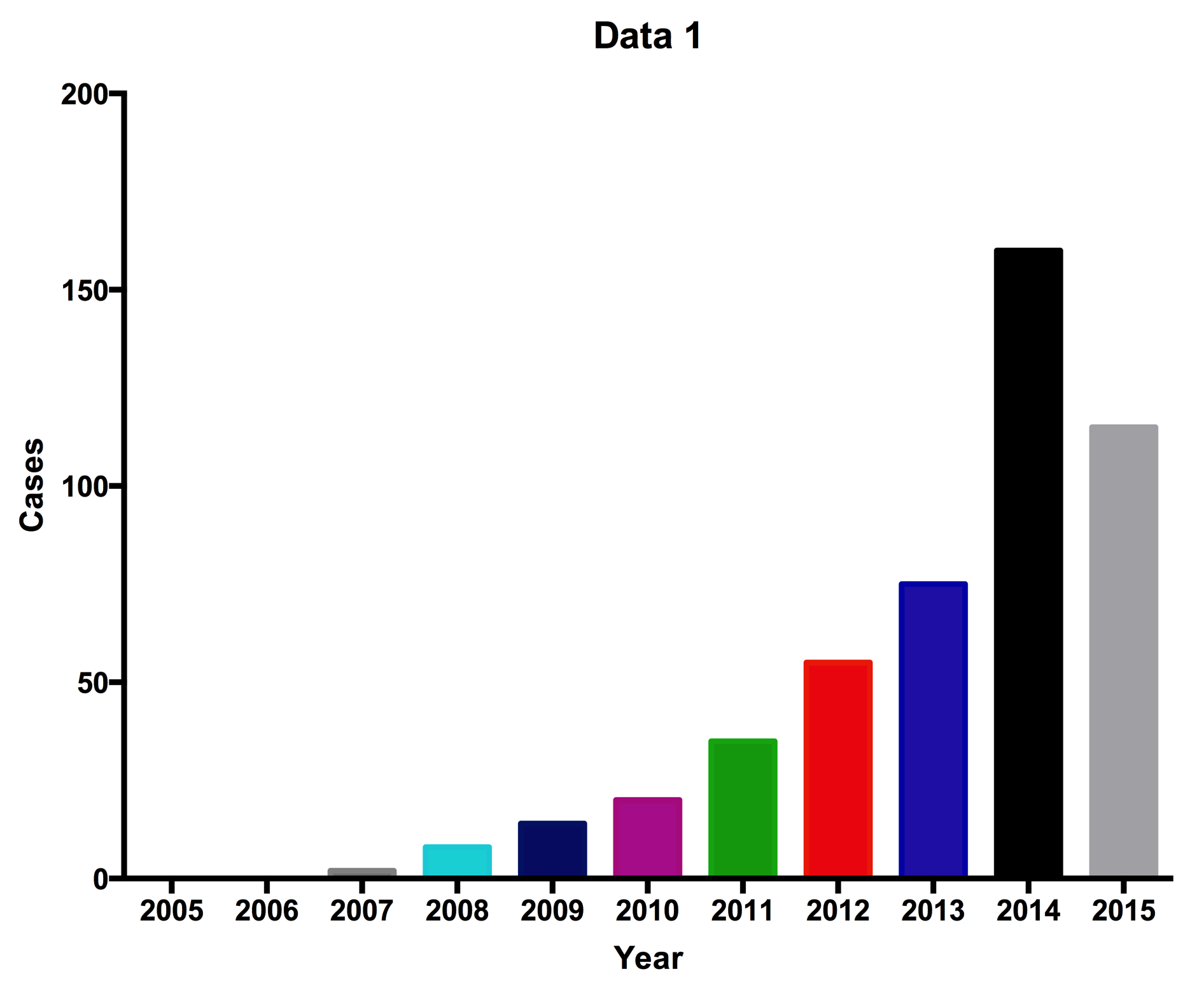
Figure 2. Clinical trials using MSCs (clinicaltrials.gov)
The number of clinical trials using MSCs dramatically increase from 2007 to date. In 2015, the number of clinical trials was recorded to June, 2016.
Although MSCs are widely used in clinical treatments, there still are some issues related to the quality and safety of MSCs. In order to maintain MSC quality and reduce the risks after MSC transplantation, MSCs should be produced in accordance with good manufacturing practice (GMP) guidelines.
Ex vivo-expanded MSCs
Media
Popular media for MSC expansion are α-minimal essential medium or Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum FCS. In recent studies, DMEM/F12 (1:1) has been used as a basal medium for MSCs, except for MSCs from umbilical cord blood, which use Iscove’s modified Dulbecco’s medium. Using these media, MSCs can be grown but with a long doubling time (more than one month) to achieve useful quantities. To stimulate MSC growth and to reduce the doubling time, cytokines or growth factors (GFs) are added to the medium. Three GFs are commonly used: epidermal growth factor (EGF), basic fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) Tarte et al., 2010 . Although these media are simple, inexpensive, and convenient for ex vivo MSC culture, they contain a xenogeneic source of GFs and proteins (from FCS) with a high risk of disease transmission. Potential risks are also related to allergies against xenoproteins and transmission of prions and viruses.
In the next generation of culture media for MSCs, FCS has been replaced by human blood-derived products. Both autologous and xenogenic blood-derived products have been successfully used for ex vivo culture of MSCs. Autologous blood-based products are optimal for ex vivo culture of MSCs for clinical application. However, it is sometimes detrimental for patients to undergo blood withdrawal. Therefore, allogeneic blood has been used as a replacement. Allogeneic samples must be subjected to serological and nucleic acid testing of blood-transmitted viruses, such as human immunodeficiency virus (HIV) and hepatitis C virus, with a supplemental step of virus inactivation before use as a supplement in culture medium.
There are two forms of blood-based products used in ex vivo culture: plasma platelet lysate (PL) and platelet rich plasma (PRP). Recent reports show that PRP is the most reliable and used product to grow MSCs from diverse sources, such as bone marrow, adipose tissue, umbilical cord, and dental pulp tissue. Only 2%–8% PRP stimulates MSC proliferation with a higher efficacy than FCS Fekete et al., 2012 . In fact, PRP is a pool of many GFs, including EGF, acidic FGF, PDGF, transforming growth factor, keratocyte growth factor, hepatocyte growth factor, and insulin-like growth factor. These are human GFs and efficiently stimulate MSCs compared with bovine GFs in FCS Fekete et al., 2012 . Studies have shown that PL- or PRP-based media efficiently maintains the phenotype and genotype of cells in long-term culture. Furthermore, the self-renewal, differentiation potential, and surface marker expression of MSCs are preserved during long-term culture in PL- or PRP-supplemented medium.
Although in vitro-cultured MSCs in media based on PL or PRP are clinically used to treat diseases via local injection or intravenous transfusion, several independent reports show that PRP or PL can drive spontaneous differentiation of MSCs in vitro. For example, Kasten et al. showed that bone marrow-derived MSCs grown in medium supplemented with PL commit to an osteoblastic lineage Kasten et al., 2008 , whereas Van Pham et al. (2013) showed that PRP drives ADSC differentiation into chondroblasts Van Pham et al., 2013 . For this reason, depending on the application, medium supplemented with PL or PRP should be carefully evaluated before use in ex vivo culture.
The third generation of media is completely defined and lacks any biological products from animal or human origins. At least five companies have successfully developed this type of medium. To replace non-defined components such as FCS, PL, and PRP, GF cocktails have been used to supplement culture media. Some of these media are produced under GMP guidelines and have obtained FDA approval as medical devices. These media also maintain the phenotypic and functional characteristics of cultured MSCs Chase et al., 2010 . The most significant problem of these media is the use of a specific protein to ensure primary cell attachment. In FCS, PL or PRP, there are proteins that facilitate MSC attachment to the surface of flasks or dishes. Conversely, for defined media, substrates must be coated to the flask/dish surface before plating MSCs to assist MSC attachment. Although xenogeneic proteins have been removed in this culture system, some coating substrates originate from animal or non-defined components.
Culture platforms
To date, there are two platforms for ex vivo culture of MSCs: monolayer and suspension culture. In both platforms, MSCs must adhere to a surface. In fact, MSCs only grow in an adherent state. In monolayer culture, MSCs are plated in flasks or dishes with a treated surface. In a clinical study, T75 or T125 flasks showed more advantages than T25 flasks. In suspension culture, MSCs adhere to microbeads suspended in media. Suspension culture of MSCs on microbeads is a new technique and easy to scale up to obtain MSCs in short-term culture Hervy et al., 2014 Wise et al., 2014 . However, most clinical applications of MSCs use monolayer culture.
Monolayer culture is the traditional technique to culture MSCs. This technique allows MSCs to proliferate for a long time without changes in MSC properties or genetic stability. Studies show that MSCs maintain their karyotype until the 25th passage Chen et al., 2014b . In another study, aneuploidy has been detected by some studies when MSCs are cultured Tarte et al., 2010 . However, they also confirmed that these modifications did not cause tumorigenesis of MSCs Chen et al., 2014a Tarte et al., 2010 Wang et al., 2013 . MSCs also maintain stemness after long-term culture with a stable phenotype, self-renewal, and differentiation potential Wang et al., 2013 . Although monolayer culture has a high risk of contamination with bacteria or fungi because of many steps during culture depending on manipulators as well as the need for a class A cabinet, monolayer culture of MSCs is the standard for ex vivo expansion of MSCs. Most expanded MSCs used in clinical applications are cultured as a monolayer. Using this technique, the quality of the flask or dish is very important. In well-treated flask surfaces, MSCs growbetter. Ventilated flasks are recommended for MSC monoculture. Closed systems for MSC expansion have been developed in recent years. Closed culture systems are considered to be optimal for clinical applications of MSCs. They can significantly decrease the number of steps, exposure to the environment, and the risk of contamination. However, not all closed systems for ex vivo cell culture satisfy GMP requirements. The first generation of closed systems was multilayer, such as CellStacks (Corning, Corning, NY, USA) or Cellfactory (Nunc, part of Thermo Fisher Scientific Inc., Waltham, MA, USA), which could be stacked in incubators. These systems increase the surface area for culture to enable the cell expansion up to 1 billion pure MSCs in 2–3 weeks Tarte et al., 2010 . However, many limitations still exist because they were not completely closed systems and required a class A cabinet for each manipulation.
The second generation is a fully closed and automated bioreactor. The main advantages of bioreactors are a large surface area to volume ratio, a closed system, automated inoculation and harvesting, and automated control of culture parameters. Terumo (Somerset, NJ, USA) has developed a fully automated bioreactor based on hollow-fiber technology to allow large-scale expansion of MSCs in a GMP-compliant system Rojewski et al., 2013 . Although this system can provide optimal tools for delivering MSCs of clinical grade, which comply with GMP, the behavior or properties of MSCs can change in this platform Guo et al., 2014 . In a recent study, a low oxygen concentration was used to maintain the growth and genetic stability of MSCs cultured in suspension culture Bigot et al., 2015 Estrada et al., 2012 Hung et al., 2012 Oliveira et al., 2012 . In another report, three-dimensional culture increased the anti-inflammatory properties of MSCs Bartosh et al., 2010 Hong et al., 2015 .
Harvesting adherent cells
MSCs must be cultured as adherent cells in both monolayer and suspension culture. After expansion, MSCs should be harvested by an enzyme. Trypsin/EDTA solution is popularly used to detach MSCs from the surfaces of culture dishes/flasks or microbeads. However, trypsin is usually derived from porcine, and not optimal for GMP production of MSCs. Some recombinant enzymes produced under GMP compliance can replace Trypsin/EDTA, such as TrypLE (Invitrogen, Thermo) and TrypZean (Sigma-Aldrich, St Louis, MO).
These second-generation enzymes are gradually being used to harvest MSCs for clinical use. Mechanical detachment using cell scrapers has also been suggested to harvest cells cultured in dishes or flasks. Although a cell scraper-based method is simple, the percentage of alive detached cells can be affected. Recently, a new de-attachment method with GMP compliance combining EDTA and chilling was patented.
Cryopreservation of cellular products
There are two forms of MSC cryopreservation. Commonly, 1.5 or 2.0 mL cryotubes are used to store MSCs in cryopreservation medium. However, a vial only holds about 1×10 7 cells which is insufficient for transplantation. In fact, for MSC transplantation, 1×10 6 cells per kg of weight is required. Therefore, similar to HSC cryopreservation, some studies have used bags for MSC cryopreservation. However, the protocols for MSC cryopreservation may be different to HSC cryopreservation. Prochymal is a commercial product containing MSCs cryopreserved in a bag, whereas Cartistem contains MSCs cryopreserved in penicillin vials.
Cryopreservation media significantly affects the quality of MSCs after thawing. They not only directly affect MSC viability but also factors affecting clinical usage. Traditionally, culture media with serum and 10% DMSO have been used in most studies. DMSO is a popular cryoprotectant. However, it also has some limitations, especially because it damages cells when presenting at high concentrations during the thawing procedure. Moreover, if DMSO is not completely removed from the cryopreserved cells, it can cause adverse reactions in patients, such as nausea, vomiting, tachycardia, bradycardia, and hypotension. Therefore, in recent years, a second generation of cryopreservation medium with other kinds of cryoprotectants has been developed, such as methylcellulose, sucrose, trehalose, glycerol, hydroxyethylstarch, polyvinylpyrrolidone, and various combinations of these cryoprotectants. However, reports show that none of these cryoprotectants are superior to DMSO. Hence, recent studies have tried to reduce the percentage of DMSO to 5% or 2%. In addition to DMSO, the serum in medium also affects MSC quality. MSCs can be well preserved in 10% DMSO and 90% FCS. However, the high ratio of animal serum can cause some adverse effects in patients. Therefore, in recent studies, FCS has been reduced to 10% or replaced with human serum. However, cryopreservation medium containing serum also has risks related to viral transmission or xenoprotein-related reactions. Auto-serum is suitable to replace animal serum or allogeneic serum. Currently, defined, serum-free and animal component-free freezing media have been developed and commercialized, such as Cryostorâ„¢ CS 10 (StemCell Technologies), Plasmalyte-A (Baxter), and Synth-a-Freeze (Gibco, Thermo).
There are two methods for freezing cryotubes for MSC cryopreservation controlled rate freezing and uncontrolled freezing (three step freezing). In the control rate freezing method, a rate of 10°C per minute has been applied with good results of viable thawed cells. The three-step freezing method involves the cells passing through three temperatures: (1) 4°C for 30–60 min, (2) −20°C for 60–120 min, and (3) −85°C overnight, and then storage in nitrogen liquid. Although control-rate freezing is clearly better than uncontrolled freezing, the most significant limitation of controlled rate freezing is the high cost of controlled rate freezing systems. At present, cryopreservation boxes have been developed. Using these boxes, the freezing rate is controlled but fixed at a specific rate. These boxes are inexpensive and can be used for MSC cryopreservation. After cryopreservation, the thawing method significantly contributes to MSC quality, especially cell viability. Commonly, MSCs are rapidly thawed by incubating the vials in a 37°C water bath for 1–2 min. The cells are then centrifuged to remove DMSO/cryoprotectants and cryopreservation medium.
Control of MSC quality
The first issue relates to MSC characteristics. Expanded MSCs should maintain their phenotypes in long-term culture. Spontaneous differentiation of MSCs always occurs during in vitro or ex vivo culture because of a heterogeneous population of MSCs. This process will proceed quickly or slowly depending on the culture conditions, especially the culture medium. Some studies have added GFs to inhibit spontaneous differentiation of MSCs. However, before application to patients, MSC characteristics must be checked.
Similar to other types of stem cells, MSCs have two important properties, self-renewal and a differentiation potential. Self-renewal is evaluated by a clonogenicity assay. This test involves seeding cells at densities of 1.5, 3, 5, and 10 cells/cm2 in a 100-mm Petri dish. It is simple, inexpensive and highly reproducible. However, the time needed for this assay is longer than the shelf-life of the final product. Therefore, this assay should be performed during evaluation of the production procedure. Although MSCs exhibit self-renewal, they also undergo senescence after long-term culture. MSCs typically proliferate for 20–50 doublings, depending on the cell source and culture conditions Cholewa et al., 2011 Izadpanah et al., 2006 Suchanek et al., 2007 . Senescent cells display aneuploidy without transformation and exhibit mutations in certain genes, such as the p53 gene Tarte et al., 2010 , upregulation of hyaluronan and proteoglycan link protein 1, keratin 18, brain-derived neurotrophic factor, or renal tumor antigen, and downregulation of pleiotrophin Schallmoser et al., 2010 . To date, senescence is easy to evaluate by a β-galactosidase staining assay.
Differentiation is also an important characteristic of MSCs. According to Dominici et al., MSCs must be able to differentiate into three kinds of mesodermal cells, namely, osteoblasts, adipocytes, and chondroblasts Dominici et al., 2006 . This suggestion has been used as a guideline to evaluate MSCs. Some reports show that senescent MSCs have a reduced differentiation potential for only osteoblasts. Differentiation assays are easy to perform with commercial differentiation kits. When cultured in inducing medium for 14–21 days, MSCs differentiate into adipocytes, osteoblasts, or chondroblasts depending on the media. Similar to self-renewal testing, differentiation potential tests are also performed for 2–3 weeks. Therefore, this test is usually applied during evaluation of the production procedure.
To evaluate MSC quality before transplantation, there are two popular indicators, surface marker expression and cell viability. Assessment of both can be carried out by flow cytometry. For cell viability, collected MSCs are stained with 7-amino-actinomycin D (7-AAD), and dead cells are identified based on the signal of 7-AAD. Although there is no guideline or regulation concerning the percentage of live MSCs for clinical grafts, most studies only use MSC samples with more than 95% live cells. In terms of surface markers for MSCs, according to Dominici et al (2006), there are two groups of markers used to confirm MSCs: positive markers (CD13, CD44, CD73, CD90, and CD105) and negative markers (CD14, CD34, CD45, and HLA-DR). Profile marker kits for these have been commercialized to confirm MSC phenotypes Dominici et al., 2006 .
Conclusion
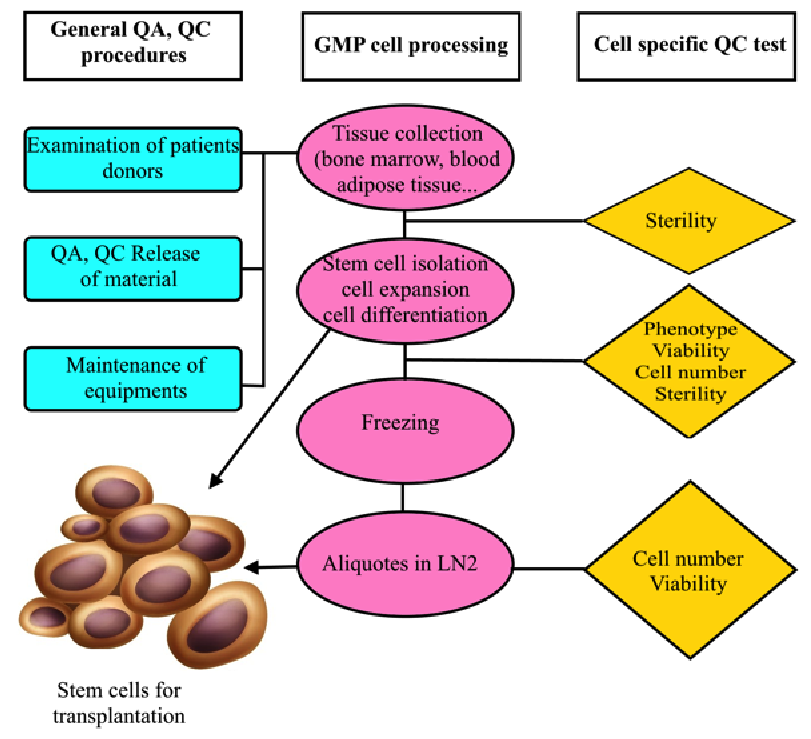
MSC production with GMP compliance ( Figure 3 ) appears to be a compelling condition to use MSCs in clinical application. GMP will maintain the quality and safety of MSCs. Clinical grade MSCs are only produced by application of regulations as well as the requirements or elements of GMP. However, all procedures should originate from clinical demands. GMP is not a standard but a set of guidelines or rules for production procedures with the highest quality and safety.
Figure 3. Flowchart of GMP-compliant production of MSCs for clinical application
All steps from donor selection to storage and delivery should be controlled and recorded.
References
- T.J. Bartosh, J.H. Ylostalo, A. Mohammadipoor, N. Bazhanov, K. Coble, K. Claypool, R.H. Lee, H. Choi, D.J. Prockop. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724-13729. Google Scholar
- M.T. Berninger, G. Wexel, E.J. Rummeny, A.B. Imhoff, M. Anton, T.D. Henning, S. Vogt. Treatment of osteochondral defects in the rabbit's knee joint by implantation of allogeneic mesenchymal stem cells in fibrin clots. J Vis Exp. 2013;:e4423. Google Scholar
- N. Bigot, A. Mouche, M. Preti, S. Loisel, M.L. Renoud, R. Le Guevel, L. Sensebe, K. Tarte, R. Pedeux. Hypoxia Differentially Modulates the Genomic Stability of Clinical-Grade ADSCs and BM-MSCs in Long-Term Culture. Stem Cells. 2015;:. Google Scholar
- T.C. Cardoso, H.F. Ferrari, A.F. Garcia, J.B. Novais, C. Silva-Frade, M.C. Ferrarezi, A.L. Andrade, R. Gameiro. Isolation and characterization of Wharton's jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnol. 2012;12:18. Google Scholar
- L.G. Chase, U. Lakshmipathy, L.A. Solchaga, M.S. Rao, M.C. Vemuri. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther. 2010;1:8. Google Scholar
- G. Chen, A. Yue, Z. Ruan, Y. Yin, R. Wang, Y. Ren, L. Zhu. Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS One. 2014a;9:e98565. Google Scholar
- G. Chen, A. Yue, Z. Ruan, Y. Yin, R. Wang, Y. Ren, L. Zhu. Monitoring the biology stability of human umbilical cord-derived mesenchymal stem cells during long-term culture in serum-free medium. Cell Tissue Bank. 2014b;15:513-521. Google Scholar
- D. Cholewa, T. Stiehl, A. Schellenberg, G. Bokermann, S. Joussen, C. Koch, T. Walenda, N. Pallua, A. Marciniak-Czochra, C.V. Suschek. Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transplant. 2011;20:1409-1422. Google Scholar
- M. Dominici, K. Le Blanc, I. Mueller, I. Slaper-Cortenbach, F. Marini, D. Krause, R. Deans, A. Keating, D. Prockop, E. Horwitz. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. Google Scholar
- J.C. Estrada, C. Albo, A. Benguria, A. Dopazo, P. Lopez-Romero, L. Carrera-Quintanar, E. Roche, E.P. Clemente, J.A. Enriquez, A. Bernad. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19:743-755. Google Scholar
- N. Fekete, M. Gadelorge, D. Furst, C. Maurer, J. Dausend, S. Fleury-Cappellesso, V. Mailander, R. Lotfi, A. Ignatius, L. Sensebe. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14:540-554. Google Scholar
- A.T. Gruia, M. Suciu, L. Barbu-Tudoran, S.M. Azghadi, M.I. Cristea, D.V. Nica, A. Vaduva, D. Muntean, A.A. Mic, F.A. Mic. Mesenchymal Stromal Cells Differentiating to Adipocytes Accumulate Autophagic Vesicles Instead of Functional Lipid Droplets. J Cell Physiol. 2015;:. Google Scholar
- L. Guo, Y. Zhou, S. Wang, Y. Wu. Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) spheroids. Journal of Cellular and Molecular Medicine. 2014;18:2009-2019. Google Scholar
- M. Hervy, J.L. Weber, M. Pecheul, P. Dolley-Sonneville, D. Henry, Y. Zhou, Z. Melkoumian. Long term expansion of bone marrow-derived hMSCs on novel synthetic microcarriers in xeno-free, defined conditions. PLoS One. 2014;9:e92120. Google Scholar
- J. Hong, J. Yun, H. Kim, S.-M. Kwon. Three-dimensional culture of mesenchymal stem cells. Tissue Eng Regen Med. 2015;12:211-221. Google Scholar
- S.P. Hung, J.H. Ho, Y.R. Shih, T. Lo, O.K. Lee. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res. 2012;30:260-266. Google Scholar
- R. Izadpanah, C. Trygg, B. Patel, C. Kriedt, J. Dufour, J.M. Gimble, B.A. Bunnell. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285-1297. Google Scholar
- P. Kasten, J. Vogel, I. Beyen, S. Weiss, P. Niemeyer, A. Leo, R. Luginbuhl. Effect of platelet-rich plasma on the in vitro proliferation and osteogenic differentiation of human mesenchymal stem cells on distinct calcium phosphate scaffolds: the specific surface area makes a difference. J Biomater Appl. 2008;23:169-188. Google Scholar
- S. Larsen, I.D. Lewis. Potential therapeutic applications of mesenchymal stromal cells. Pathology. 2011;43:592-604. Google Scholar
- H.M. Lee, B.S. Joo, C.H. Lee, H.Y. Kim, J.H. Ock, Y.S. Lee. Effect of Glucagon-like Peptide-1 on the Differentiation of Adipose-derived Stem Cells into Osteoblasts and Adipocytes. J Menopausal Med. 2015;21:93-103. Google Scholar
- S.M. Melief, J.J. Zwaginga, W.E. Fibbe, H. Roelofs. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455-463. Google Scholar
- F. Montespan, F. Deschaseaux, L. Sensebe, E.D. Carosella, N. Rouas-Freiss. Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: implications in bone repair therapy. J Immunol Res. 2014;2014:230346. Google Scholar
- P.H. Oliveira, J.S. Boura, M.M. Abecasis, J.M. Gimble, C.L. da Silva, J.M. Cabral. Impact of hypoxia and long-term cultivation on the genomic stability and mitochondrial performance of ex vivo expanded human stem/stromal cells. Stem Cell Res. 2012;9:225-236. Google Scholar
- F. Perdisa, N. Gostynska, A. Roffi, G. Filardo, M. Marcacci, E. Kon. Adipose-Derived Mesenchymal Stem Cells for the Treatment of Articular Cartilage: A Systematic Review on Preclinical and Clinical Evidence. Stem Cells Int. 2015;2015:597652. Google Scholar
- M.T. Rojewski, N. Fekete, S. Baila, K. Nguyen, D. Furst, D. Antwiler, J. Dausend, L. Kreja, A. Ignatius, L. Sensebe. GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device quantum cell expansion system. Cell Transplant. 2013;22:1981-2000. Google Scholar
- M. Saeidi, A. Masoud, Y. Shakiba, J. Hadjati, M. Mohyeddin Bonab, M.H. Nicknam, M. Latifpour, B. Nikbin. Immunomodulatory effects of human umbilical cord Wharton's jelly-derived mesenchymal stem cells on differentiation, maturation and endocytosis of monocyte-derived dendritic cells. Iran J Allergy Asthma Immunol. 2013;12:37-49. Google Scholar
- K. Schallmoser, C. Bartmann, E. Rohde, S. Bork, C. Guelly, A.C. Obenauf, A. Reinisch, P. Horn, A.D. Ho, D. Strunk. Replicative senescence-associated gene expression changes in mesenchymal stromal cells are similar under different culture conditions. Haematologica. 2010;95:867-874. Google Scholar
- J. Shao, W. Zhang, T. Yang. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res. 2015;48:62. Google Scholar
- T. Squillaro, G. Peluso, U. Galderisi. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2015;:. Google Scholar
- J. Suchanek, T. Soukup, R. Ivancakova, J. Karbanova, V. Hubkova, R. Pytlik, L. Kucerova. Human dental pulp stem cells--isolation and long term cultivation. Acta Medica (Hradec Kralove). 2007;50:195-201. Google Scholar
- K. Tarte, J. Gaillard, J.J. Lataillade, L. Fouillard, M. Becker, H. Mossafa, A. Tchirkov, H. Rouard, C. Henry, M. Splingard. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549-1553. Google Scholar
- P. Van Pham, K.H. Bui, D.Q. Ngo, N.B. Vu, N.H. Truong, N.L. Phan, D.M. Le, T.D. Duong, T.D. Nguyen, V.T. Le. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther. 2013;4:91. Google Scholar
- Y. Wang, Z. Zhang, Y. Chi, Q. Zhang, F. Xu, Z. Yang, L. Meng, S. Yang, S. Yan, A. Mao. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013;4:e950. Google Scholar
- J.K. Wise, A.I. Alford, S.A. Goldstein, J.P. Stegemann. Comparison of uncultured marrow mononuclear cells and culture-expanded mesenchymal stem cells in 3D collagen-chitosan microbeads for orthopedic tissue engineering. Tissue Eng Part A. 2014;20:210-224. Google Scholar
 Biomedpress
Biomedpress Open Access
Open Access 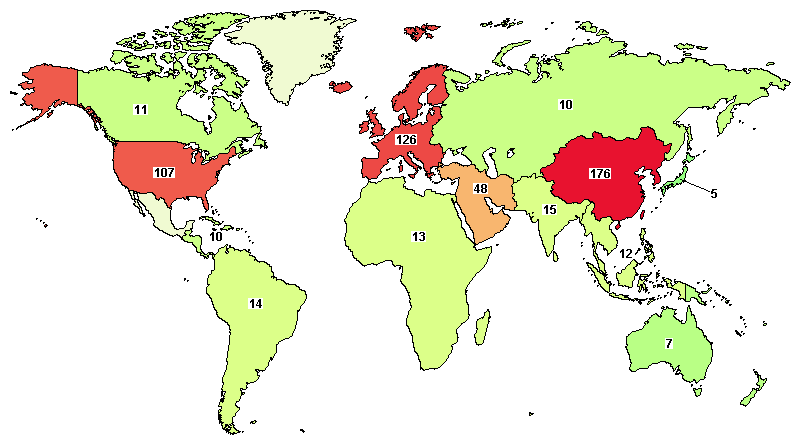
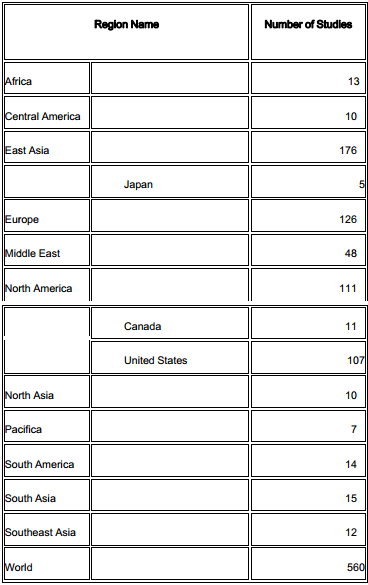







There are more than 500 clinical trials registered at clinicaltrials.gov. Many clinical trials are being performed in East Asia and North America.