Abstract
Bone marrow mesenchymal stem cells (BM-MSCs) are multipotent stem cells that can differentiate into some kinds of mesenchymal cells such as osteoblasts, chondroblasts and adipocytes. These cells were discovered for a long time and considered as the first discovered source of MSCs. BM-MSC transplantation was used to treat some diseases such as bone disease, myocardial infarction, stroke, diabetes mellitus… This study aimed to provide a new method to in vitro primarily culture and secondary culture of BM-MSCs that were compliant with good manufacturing practice for clinical applications. Bone marrow was aspirated from the hip bone using special needles and syringes. Mononuclear cells (MNCs) were isolated from bone marrow by BM-MSC extraction kit. These MNCs were cultured in the commercial medium – MSC-Cult supplemented with 10% activated platelet rich plasma (aPRP) to obtain BM-MSCs. The results showed that 100% samples of bone marrow can be successfully cultured to produce BM-MSCs. Obtained cells exhibited the MSC phenotypes, and maintained the stable and normal karyotype up to 10th passages, and failed to cause tumor in the mouse models. This research provided an advanced protocol for clinical applications of BM-MSCs.
Introduction
Bone marrow-derived mesenchymal stem cells (BM-MSCs) have long been used in both preclinical and clinical studies. BM-MSCs were first discovered by Friedenstein in 1968 Friedenstein et al., 1968 . Based on the first demonstration by Friedenstein, these cells were also seen in other tissues such as umbilical cord blood Pham et al., 2014 , umbilical cord Leite et al., 2014 , adipose tissue Van Pham et al., 2013 , dental pulp Ferro et al., 2014 , placenta Brooke et al., 2009 , and menstrual blood de Carvalho Rodrigues et al., 2012 . To confirm which stem cells are MSCs, Dominici et al. (2006) suggested three criteria: plastic adherence when maintained in standard culture conditions, expression of CD105, CD73, and CD90 and non-expression of CD45, CD34, CD14, or CD11b, CD79 alpha, or CD19 and HLA-DR, and successful differentiation into osteoblasts, adipocytes, and chondroblasts in vitro Dominici et al., 2006 .
BM-MSCs are used to pre-clinically treat diseases in animal models of Alzheimer’s and Parkinson’s diseases Danielyan et al., 2014 Danielyan et al., 2011 , rheumatoid arthritis Papadopoulou et al., 2012 , amyotrophic lateral sclerosis Chan-Il et al., 2013 , diabetes Ezquer et al., 2009 , etc. They are used clinically in humans to treat idiopathic pulmonary fibrosis Chambers et al., 2014 , cirrhosis (hepatocellular carcinoma) Vainshtein et al., 2014 , cirrhotic rats Li et al., 2013 , autoimmune diseases Lee et al., 2014 , cartilage disease Veronesi et al., 2013 , etc.
To increase the number of BM-MSCs, BM-MSCs are subjected to long-term culture in vitro. However, in most studies, MSCs were cultured in media containing xenogeneic additives such as fetal bovine serum (FBS) Choudhery et al., 2013 Huang et al., 2014 Odabas et al., 2014 . These media are associated with risks such as prion-mediated infections, viral transmission, and adverse immunological reactions. However, serum-free media are commercially available for use in BM-MSC culture. The main limitations of these media are their high cost and complexity. In fact, when grown in serum-free media, BM-MSCs hardly adhere to culture-flask surfaces; hence, all culture flasks must be pre-coated with adherent matrixes.
In this study, we established an animal product-free expansion protocol by using autologous activated platelet rich plasma. During the procedure, all components of animal origin, such as trypsin, were not used. Thus, because this protocol is free of animal products, it is safe and feasible for large-scale BM-MSC isolation and expansion for use in clinical applications.
Materials-Methods
Bone Marrow and Peripheral Blood Collection
The project was approved by the local ethics committee. Five donors participated in this study. In total, 20 mL of bone marrow was aspirated from each donor after obtaining informed consent. The collection was performed in accordance with the standards of the local ethics committee. Besides BM samples, 20 mL peripheral blood was also collected from each donor. Both BM and peripheral blood were anticoagulated by using CDPA solution. All samples were immediately transferred to the laboratory.
MNC Isolation and Activated PRP Preparation
Mononuclear cells (NMCs) were isolated from the bone marrow. The BM samples were diluted at a ratio of 1:1 with phosphate-buffered solution (PBS) and then subjected to density centrifugation using Ficoll-Hypaque (1.077 g/mL; Sigma-Aldrich, St Louis, MO, USA). BM samples were centrifuged at 3000 rpm for 30 min. MNCs were collected from the interphase of the centrifuge tube. The collected MNCs were washed twice with PBS and then used for further experiments.
Peripheral blood samples were used to produce activated PRP (aPRP). ACD anti-coagulated peripheral blood samples were centrifuged in two steps to get PRP. In the first step, these samples were centrifuged at 500 × g for 5 min to obtain plasma. In the second step, the plasma samples were centrifuged at 800 × g for 10 min to obtain platelet pellets at the bottom of the tubes. To prepare aPRP, a third of the plasma volume and the platelet pellet was collected and re-suspended, following which 100 μL CaCl 2 per 1 mL of PRP was added to activate growth factor release. The samples were then incubated at 37°C for 30 min or until clotting occurred.
Primary Culture
Primary culture was performed as described in a previously published study Pham et al., 2014 . Five BM samples were used for primary culture. MNCs were cultured in DMEM/F12 medium containing 1% antibiotic-antimycotic (Sigma-Aldrich) and various concentrations of autologous aPRP (2%, 5%, 7%, or 10%) or 10% fetal bovine serum (FBS) for the control. The cells were plated at a density of 5 × 10 4 cells/mL in T-75 flasks (Corning) and incubated at 37°C with 5% CO2. After three days of culture, 6 mL of fresh medium was added to each T-75 flask. The medium was replaced with fresh medium every 4 days until the cells reached 70–80% confluence. The efficiency of the media was evaluated by considering the time required for adherent cells to appear and reach 70– 80% confluence for the first subculture.
Secondary Culture
After successful primary culture, the samples were sub-cultured to evaluate the effects of various media. The proliferation rate was evaluated by the XCELLIgence system (Roche Applied Science, Indianapolis, IN, USA). A total of 1 × 10 3 cells were seeded into each well of a 96-well E-plate in triplicate. The culture plates were placed into the XCELLIgence system and incubated at 37°C in the presence of 5% CO2. Cell proliferation was monitored for 300 h, with the medium being replaced every third day. Both the cell doubling time and slope value were determined by a software of the XCELLIgence system.
Flow Cytometry
Cell markers were analyzed by following a previously published protocol. Briefly, cells were washed twice in PBS containing 1% bovine serum albumin (Sigma-Aldrich). The cells were then stained with anti-CD13-FITC, anti-CD14-FITC, anti-CD34-FITC, anti-CD44-PE, anti-CD45-FITC, anti-CD73-FITC, anti-CD90-PE, anti-CD105-FITC, anti-CD106-PE, anti-CD166-PE, or anti-HLA-DR-FITC antibodies (all purchased from BD Biosciences, San Jose, CA, USA). Stained cells were analyzed by FACSCalibur flow cytometer (BD Biosciences). Isotype controls were used in all analyses.
In Vitro Differentiation
For differentiation into adipogenic cells, BM-MSCs were differentiated as described previously. Briefly, passage five cells were plated at a density of 1 × 10 4 cells/well in 24-well plates. At 70% confluence, the cells were cultured for 21 days in DMEM/F12 containing 0.5 mmol/L 3-isobutyl-1-methyl-xanthine, 1 nmol/L dexamethasone, 0.1 mmol/L indomethacin, and 10% FBS (all purchased from Sigma-Aldrich). Adipogenic differentiation was evaluated by observing lipid droplets in cells, stained with Oil Red, under a microscope.
For differentiation into osteogenic cells, BM-MSCs were plated at a density of 1 × 10 4 cells/well in 24-well plates. At 70% confluence, the cells were cultured for 21 days in DMEM/F12F12 containing 10% FBS, 10 -7 mol/L dexamethasone, 50 μmol/L ascorbic acid-2 phosphate, and 10 mmol/L β-glycerol phosphate (all purchased from Sigma-Aldrich). Osteogenic differentiation was confirmed by Alizarin red staining.
Tumorigenicity Assay
The tumorigenicity of BM-MSCs was examined in athymic nude mice. All manipulations of mice were approved by the Local Ethics Committee of Stem Cell Research and Application, University of Science (Ho Chi Minh City, Vietnam). Each mouse was injected subcutaneously with 5 × 10 6 cells (three mice per group). As a positive control, the mice were also injected with breast cancer cells at a different site. Tumor formation in mice was followed up for three months.
Statistical Analysis
The significance of differences be tween mean values was assessed by t-tests and analysis of variance. A P-value of less than 0.05 was considered to be significant. All data were analyzed by Prism 6 software.
Results
Bone Marrow Isolation and Primary Culture
By daily observation under an inverted microscope, the adherent cells were found to appear sooner in groups 10% FBS an d 10% PRP on day 2 than in groups 2.5% P RP and 5% PR P on day 3. Therefore, the figures capture d on day 3 show that a few a dherent cells appeared on the flask surfaces. Figure 1 shows that there w ere fewer cell s appearing in the same microscopic field in groups treated with a lower concentration of PRP. After 10 days, cells reached approximately 70% confluence in almost samples of four groups. These cells were continuously sub-cultured for four passages. The cells in th is passage were used for further experiments such as evaluation of surface marker expression,cell proliferation, cell differentiation, karyotyping, and tumorigenicity.
Figure 1. Primary culture of MNCs in four kinds of medium
Expression of Surf ace Markers in Cells of t he Four Groups
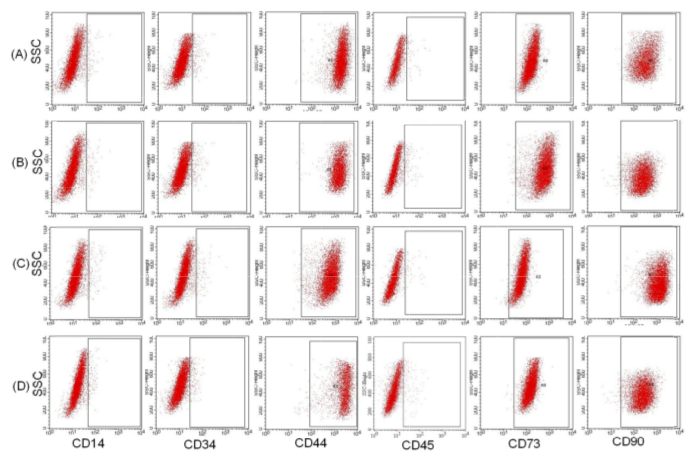
The results are presented in Figure 2 . These results showed that MSCs cultured in four different media expressed similar surface markers of MSCs. The MSCs of these four groups w ere strongly positive for CD4 4, CD73, and C D90; 95.5%, 98.71%, and 99 .12%, respectively, for group 10% FBS; 96.21%, 97 .19%, and 98.31%, respectively, for group 2.5% PRP; 97.31%, 96.78%, and 97.18 %, respectively, for group 5% PRP; and 97.31%, 95.81 %, and 96.17 % respectively, for group 10% PRP. The differences between the percentages of positive markers were non- significant. Moreover, the MSCs in these four groups w ere also similar with respect to the non-expression of markers such as CD14, CD 34, and CD45. In fact, there were only 2 .13%, 1.18%, 2.11%, 1.11% CD14+ cells, 0.11%, 0.45% , 0.31%, 0.19 % CD34+ cells, and 0.33%, 0.31%, 0.71%, 0.49% CD45+ cells, respectively, for groups 10% FBS, 2.5% PRP, 5 % PRP, and 10 % PRP.
Figure 2. Expression of mesenchymal stem cell markers in cells of the four different groups
Cells in the four groups expressed CD44 , CD73, CD90 , and CD105 and were negative for CD14, CD34, CD45, and HLA-DR. Line A: group 10% FB S, line B: group 2.5% PRP; line C: group 5 % PRP, and line D: group 10% PRP.
Differentiation Potential of BM- MSCs in the F our Different Groups
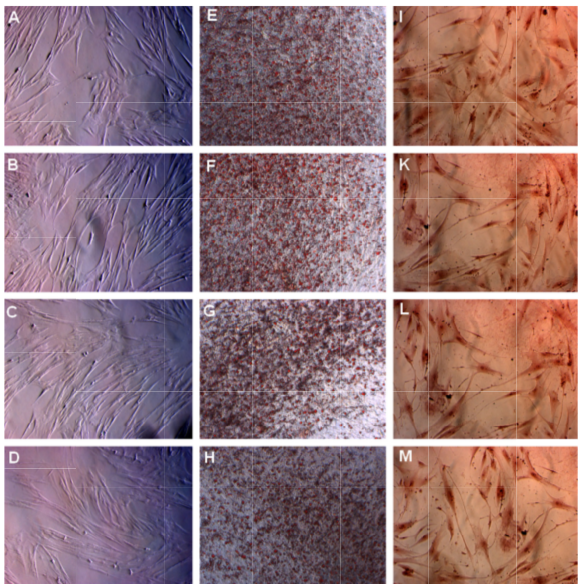
BM-MS Cs in the four groups successfully differentiated int o both adipocytes and osteoblasts. After induction with ad ipogenesis-inducing medium, BM-MSCs in the four group s accumulated lipid droplets i n the cellular cytosol from day 14; this coul d be visualize d under an inverted microscope ( Figure 3 ). The lipid droplts gradually increased in size, and at day 21, these droplets could clearly be visualized and stained red with Oil Red ( Figure 3 ). Similarly, the shape of these cells also change d when incubated in osteog enesis-inducing medium. After 14 days of induction, BM -MSCs exhibited the characteristic longer shape associated with fibroblasts ( Figure 3 ). More importantly, these induced cells stained positive with Alizarin Red.
Figure 3. MSC s in the four groups successfully differentiated into adipocytes and osteoblasts in vitro
MSCs accumulated lipids in the cell vacuole and formed lipid droplets that were stained with Oil Red (E, F, G, and H depict groups 10 % FBS, 2 .5% PRP, 5% PRP, and 10 % PRP, respectively). MSCs also stored Ca2+ as an oste oblast property that stained positive with Alizarin Re d (I, K, L, and M depict groups 10% FBS, 2.5% PRP, 5% PRP, and 10 % PRP, respectively). Magnification 100X
Proliferation of BM-M SCs in the Four different Media
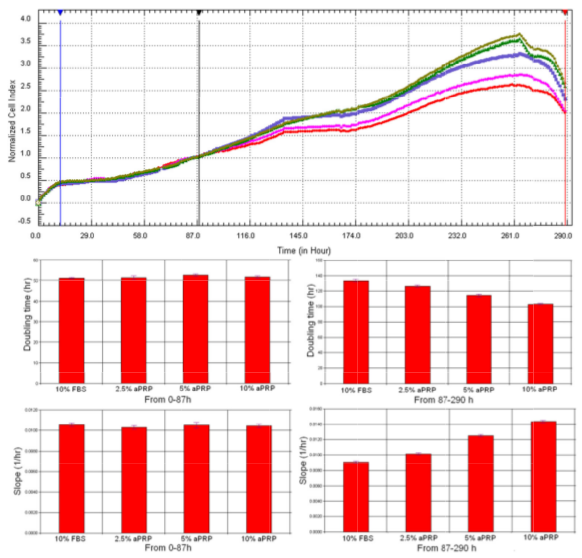
Proliferation rates of BM-MSCs w ere different among the four media. Base d on the proliferation curve depicted in Fig. 4A, the results showed t hat BM-MSC proliferation in the 10% FBS group was significantly slower than that in 5% PRP and 10% PRP groups.
However, it was equivalent to that observed in 2.5% PRP group. These observations w ere confirmed by doubling time and slope value analysis ( Figure 4 ). As presented in Figure 4 , from 0h t o 87h, the doubling time and slope values w ere similar in four groups; however, from 87h to 290h, the doubling time of BM-MSCs in groups 5% P RP and 10% PRP was significantly reduced compare d to the doubling time in groups 2.5% PRP and 10 % FBS. Doubling time of BM -MSCs in 10 % FBS and 2. 5% PRP groups was similar. In contrast to the doubling time values , slope values gradually increased from group 10% FBS, 2.5% PRP, 5% PRP, and 10 % PRP. These results were clearly different from those of the control (blank wells).
Figure 4. Proliferation of BM-MSCs in the four different media
BM-MSC proliferation in group 10% FBS was similar to that in group 2.5% PRP, but slower than that observed in groups 5% PRP and 10% PRP (A). Red line: 10% FBS, Pink line: 2.5% aPRP, Violet line: 7.5% aPRP; Green and grey lines: 10% aPRP.
BM-MSCs Maint ained Normal Karyotypes
BM-MS Cs of the four groups at 10th passage we re used for karyotype analysis. The results showed that at the 10th passage, the BM-MSCs in all the four groups (10% FBS, 2.5 % PRP, 5% PRP, and 10% PRP) maintained the normal karyotype (2n = 46 chromosomes).
Tumorigenesis of BM-MSCs in Athymic Mice
BM-MS Cs cultured in the various media were injected into athymic nude mice. As a positive control, breast cancer cells w ere injected a t a different location. The results showed th at BM-MSCs could not form tumors in athy mic nude mice , while breast cancer cells formed tumors in the control group after one month.
Discussion
BM-MSCs are the most popular sou rce of mesenchymal stem cells used in clinical applicati ons. To date, b oth autologous and allogeni c BM-MSCs ar e used for the treatment of diseases. For example, Prochymal TM product obtained from BM-MSCs, is approved as a stem cell drug for the treatment of graft versus host disease (GVHD) Kebriaei et al., 2009 Vaes et al., 2012 . In the clinicaltrial.gov database, there were more than 200 registered trials with about 10 kinds of diseases that have been treated by BM-MSC transplantation. Therefore, expansion of BM-MSCs for both autologous and allogenic transplantation is essential for the application of stem cells in the treatment of diseases.
In this study, we successfully cultured BM-MSCs in xenogeneic protein-free conditions. In most previously published studies, BM-MSCs were cultured in media supplemented with FBS or FCS. FBS and FCS contained several xenogeneic proteins as well as infectious agents, including viruses and prions. Therefore, BM-MSCs could be contaminated with these components of bovine serum. After transplantation, these components could elicit an immune response in the recipient or transmit viruses or prions to cause critical diseases in human, which is riskier. By replacement of FBS or FCS with PRP, BM-MSCs can be cultured in conditions that are entirely free of bovine serum.
However, the next question to be addressed was whether cultivation of BM-MSCs in PRP-supplemented medium could cause changes in the BM-MSCs. In the next experiment, we evaluated the phenotype, differentiation potential, as well as karyotype of BM-MSCs. BM-MSCs were cultured in PRP-supplemented medium well-done conserved MSC properties. Similar to BM-MSCs cultured in FBS medium, MSCs in PRP medium satisfied with minimum criteria of MSCs that Dominici et al. suggested in 2006 Dominici et al., 2006 . In fact, these cells were positive for CD44, CD73, CD90 and CD105 and negative for CD14, CD34, CD45, and HLA-DR. This phenotype also agreed with that reported in previously published studies Iudicone et al., 2014 Narbona-Carceles et al., 2014 Robey et al., 2014 .
Although culture medium with 10% FBS excellently enhanced cell adherence to the flask surface in a manner similar to that observed in culture medium with 10% PRP during the primary culture, during the secondary culture, PRP efficiently stimulated BM-MSC growth. In fact, at 2.5% PRP concentration in the culture medium, proliferation rates of BM-MSCs in both 2.5% PRP medium and 10% FBS medium were similar. Moreover, their proliferation rates were significantly different between 10% FBS medium and 5% PRP and 10% PRP media. These results showed that PRP is a rich source of natural human growth factors. Moreover, these growth factors triggered BM-MSC growth. PRP also strongly stimulated the proliferation of MSCs that originated from other tissues such as umbilical cord blood Murphy et al., 2012 Pham et al., 2014 , adipose tissue Atashi et al., 2014 Van Pham et al., 2014 , and human dental stem cells Lee et al., 2011 . The last criterion evaluated was the differentiation potential of BM-MSCs into mesenchymal cells such as adipocytes, osteoblasts, and chondrocytes. The BM-MSCs cultivated in PRP medium and FBS medium could differentiate into adipocytes and osteoblasts. BM-MSCs cultured in PRP-supplemented medium were also found to maintain the MSC phenotype.
To satisfy the stem cells for transplantation, BM-MSCs cultured in PRP medium were examined for changes in the normal karyotype and for their tumorigenicity. The results demonstrated that PRP did not affect the karyotype of BM-MSCs. At the 10th passage, BM-MSCs in both PRP- or FBS-supplemented media maintained the normal karyotypes (2n = 46). Owing to the normal karyotype, these cells also could not form tumors in the athymic mice. Considering these analysis results, PRP could replace FBS in BM-MSC culture and satisfy the criteria of MSCs used for clinical transplantation.
Conclusion
BM-MSCs are important autologous stem cells for regenerative medicine. This study provided a simple protocol to isolate and culture BM-MSCs for clinical applications. Owing to supplementation with PRP, the BM-MSC culture medium became free of xenogeneic proteins, foreign viruses, and other infectious agents. Similar to BM-MSC cultured in FBS-supplemented medium, BM-MSCs cultured in PRP-supplemented medium maintained their phenotype, differentiation potential, as well as conserved their normal karyotype after 10 passages and did not form tumors in mice. This study also suggested that BM-MSCs could be cultured in a medium supplemented with 2.5% PRP. Considering these results, expanded BM-MSCs can satisfy the criteria underlying good manufacturing practice standards for clinical usage.
Abbreviations
aPRP: Activated platelet rich plasma; BM-MSC: Bone marrow mesenchymal stem cells; FBS: Fetal bovine serum; GVHD: Graft versus host disease; MNCs: Mononuclear cells: PBS: Phosphate buffer saline
References
- F. Atashi, M.E. Jaconi, B. Pittet-Cuenod, A. Modarressi. Autologous Platelet-Rich Plasma: A Biological Supplement to Enhance Adipose-Derived Mesenchymal Stem Cell Expansion. Tissue engineering Part C, Methods 10.1089/ten.TEC.2014.0206. 2014;:. Google Scholar
- G. Brooke, T. Rossetti, R. Pelekanos, N. Ilic, P. Murray, S. Hancock, V. Antonenas, G. Huang, D. Gottlieb, K. Bradstock. Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. British journal of haematology. 2009;144:571-579. Google Scholar
- D.C. Chambers, D. Enever, N. Ilic, L. Sparks, K. Whitelaw, J. Ayres, S.T. Yerkovich, D. Khalil, K.M. Atkinson, P.M. Hopkins. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology (Carlton, Vic). 2014;19:1013-1018. Google Scholar
- C. Chan-Il, L. Young-Don, K. Heejaung, S.H. Kim, H. Suh-Kim, S.S. Kim. Neural induction with neurogenin 1 enhances the therapeutic potential of mesenchymal stem cells in an amyotrophic lateral sclerosis mouse model. Cell transplantation. 2013;22:855-870. Google Scholar
- M.S. Choudhery, M. Badowski, A. Muise, D.T. Harris. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy. 2013;15:330-343. Google Scholar
- L. Danielyan, S. Beer-Hammer, A. Stolzing, R. Sch Fer, G. Siegel, C. Fabian, P. Kahle, T. Biedermann, A. Lourhmati, M. Buadze. Intranasal delivery of bone marrow derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer?s and Parkinson?s diseas. . Cell transplantation 10.3727/096368914x684970. 2014;:. Google Scholar
- L. Danielyan, R. Schafer, A. von Ameln-Mayerhofer, F. Bernhard, S. Verleysdonk, M. Buadze, A. Lourhmati, T. Klopfer, F. Schaumann, B. Schmid. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation research. 2011;14:3-16. Google Scholar
- D. Carvalho Rodrigues, K.D. Asensi, L. Vairo, R.L. Azevedo-Pereira, R. Silva, E. Rondinelli, R.C. Goldenberg, A.C. Campos de Carvalho, T.P. Urmenyi. Human menstrual blood-derived mesenchymal cells as a cell source of rapid and efficient nuclear reprogramming. Cell transplantation. 2012;21:2215-2224. Google Scholar
- M. Dominici, K. Le Blanc, I. Mueller, I. Slaper-Cortenbach, F. Marini, D. Krause, R. Deans, A. Keating, D. Prockop, E. Horwitz. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. Google Scholar
- F. Ezquer, M. Ezquer, V. Simon, F. Pardo, A. Yanez, D. Carpio, P. Conget. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:1354-1365. Google Scholar
- F. Ferro, R. Spelat, C.S. Baheney. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. Methods in molecular biology (Clifton, NJ). 2014;1210:91-115. Google Scholar
- A.J. Friedenstein, K.V. Petrakova, A.I. Kurolesova, G.P. Frolova. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. Google Scholar
- J. Huang, H. Sha, G. Wang, G. Bao, S. Lu, Q. Luo, Q. Tan. Isolation and characterization of ex vivo expanded mesenchymal stem cells obtained from a surgical patient. Molecular medicine reports 10.3892/mmr.2014.2892. 2014;:. Google Scholar
- P. Iudicone, D. Fioravanti, G. Bonanno, M. Miceli, C. Lavorino, P. Totta, L. Frati, M. Nuti, L. Pierelli. Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. Journal of translational medicine. 2014;12:28. Google Scholar
- P. Kebriaei, L. Isola, E. Bahceci, K. Holland, S. Rowley, J. McGuirk, M. Devetten, J. Jansen, R. Herzig, M. Schuster. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:804-811. Google Scholar
- H.K. Lee, S.H. Lim, I.S. Chung, Y. Pa rk, M.J. Park, J.Y. Kim, Y.G. Kim, J.T. Hong, Y. Kim, S.B. Han. Preclinical efficacy and me chanisms of mes enchymal stem cells in animal m odels of autoim mune diseases. Immune netwo rk. 2014;14:81-88. Google Scholar
- J.Y. Lee, H . Nam, Y.J. Park, S.J. Le e, C.P. Chung, S.B. Han, G. Lee. The effects o f platelet-rich plasma derived from human umbilical cord blood on the osteogenic differentiation of human dental stem cells. In vitro cellular & developmental biology Animal. 2011;47:157-164. Google Scholar
- C. Leite, N.T. Silva, S. Mendes, A. Ribeiro, J.P. de Faria, T. Lourenco, F. Dos Santos, P.Z. Andrade, C.M. Cardoso, M. Vieira. Differentiation of human umbilical cord matrix mesenchymal stem cells into neural-like progenitor cells and maturation into an oligode droglial-like line age. PloS one. 2014;9:e111059. Google Scholar
- T. Li, J. Zhu, K. Ma, N. Liu, K. Feng, X. Li, S. Wang, P. Bie. Autologous bone marrow-derived mesenchymal stem cell transplantation promotes liver regeneration after portal vein embolization in cirrhotic rats. The Journal of surgical research. 2013;184:1161-1173. Google Scholar
- M.B. Murphy, D. Blashki, R.M. Buchanan, I.K. Yazdi, M. Ferrari, P.J. Simmons, E. Tasciotti. Adult and umbilical cord blood-derived platelet-rich plasm a for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservatio n. Biomaterials 3. 2012;3:5308-5316. Google Scholar
- J. Narbona-Carceles, J. Vaquero, S.S. B, F. Forriol, M.E. Fernandez-Santos. Bone marrow mesenchymal stem cell aspirates from alternati ve sources Is the knee as good as the iliaccrest?. Injury 45 Suppl. 2014;4:S42-47. Google Scholar
- S. Odabas, A.E. Elcin, Y.M. Elcin. Isolation and characterization of mesenchy mal stem cells. Methods inmolecular biology (Clifton, NJ). 2014;1109:47-63. Google Scholar
- A. Papadopoulou, M . Yiangou, E. Athanasiou, N. Zogas, P. Kaloyannidis, I. Batsis, A. Fassas, A. Anagnostopoulos, E. Yannaki. Mesenchymal stem cells are conditionally therapeutic in preclinical models of rheumatoid arthritis. Annals of the rheumatic diseases. 2012;71:1733-1740. Google Scholar
- P.V. Pham, N.B. Vu, V .M. Pham, N. H . Truong, T.L. Pham, L.T. Dang, T.T. Nguyen, A.N. Bui, N.K. Phan. Good manufacturing ractice-compli ant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. Journal of translational medicine. 2014;12:56. Google Scholar
- P.G. Robey, S.A. Kuznetsov, J. Ren, H.G. Klein, D. F. Stroncek. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone 10.1016/j.bone.20 14.07.020. 2014;:. Google Scholar
- B. Vaes, W. Van'tHof, R. Deans, J. Pinxte ren. Application of MultiStem((R)) Allogeneic Cells for Immunomodulatory Therapy: Clinical Progress and Pre-Clinical Challenges in Prophylaxis for Graft Versus Host Disease. Frontiers in immunology. 2012;3:345. Google Scholar
- J.M. Vainshtein, R. Kabarriti, K.J. Mehta, J. Roy-C howdhury, C. Guha. Bone marrow-derived stromal cell therapy in cirrhosis: clinical evide nce, cellular mechanisms, and implications for the treatment of hepatocellular carcinoma. International journal of radiation. 2014;oncology:biology, physics 89, 7 86-803. Google Scholar
- P. Van Pham, K.H. Bui, D.Q . Ngo, N.B. Vu, N.H. Truong, N.L. Phan, D.M. Le, T.D. Duong, T.D. Nguyen, V.T. Le. Activated platele t-rich plasmai proves adipos e-derived stem cell transplantation efficiency in injured articular cartilage. Stem cell research & therapy. 2013;4:91. Google Scholar
- P. Van Pham, N. B. Vu, N.L.-C . Phan, D.M. Le, N.C. Truong, N.H. Truong, K.H.-T. Bui, N.K. Ph an. Good manufacturing practice-compliant isolation and culture of human adipose derived stem cells. Biomed Res Ther. 2014;1:1-9. Google Scholar
- F. Veronesi, G. Giavaresi, M. Tschon, V. Borsari, N. Nicoli Aldini, M. Fini. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesen chymal stem cells in cartilage disease. Stem cells and development. 2013;22:181-192. Google Scholar
 Biomedpress
Biomedpress Open Access
Open Access 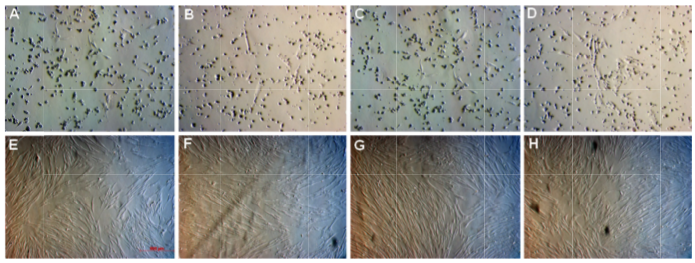







A-D: image s of the samples were captured under an inverted microscope at day 3 for group 10% FBS (A), 2.5% PRP (B), 5% PRP (C), and 10% PRP (D). E-H: images of the samples were captured under an inverted microscope at day 10 for group 10% FBS (E ), 2.5% PRP (F), 5% PRP (G), and 10% PRP (H). Magnification 100X.