Abstract
Introduction: Embryonic stem cells are pluripotent, thus capable of differentiating into all types of cells derived from the three germ layers. However, the application of embryonic stem cells (ESCs) for preclinical and clinical studies is difficult due to ethical concerns. Induced pluripotent stem cells (iPSCs) are derived from differentiation and have many ESC characteristics. The study herein examines the production of iPSCs from reprogramming of mouse embryonic fibroblasts (MEFs) via transduction with defined factors.
Methods: MEFs were collected from mouse embryos via a previously published protocol. The cells were transduced with a single polycistronic viral vector encoding mouse cDNAs of Oct3/4, Sox2, Klf4 and c-Myc. Transduced cells were treated and sub-cultured with ESC medium. The cells were evaluated as iPSCs with specific morphology, and expression SSEA-1, Oct3/4, Sox2 and Nanog. In addition, they also were evaluated for pluripotency by assessing alkaline phosphatase (AP) activity and in vivo teratoma formation.
Results: Under the reprogrammed conditions, the transduced cells displayed a change in morphology, forming ESC-like clusters. These cell clusters strongly expressed pluripotent markers as well as ESC-specific genes. Furthermore, the colonies exhibited higher AP activity and formed teratomas when injected into the murine testis.
Conclusions: The study herein suggests that MEFs can be reprogrammed into iPSCs using a polycistronic viral vector encoding mouse cDNAs for Oct3/4, Sox2, Klf4 and c-Myc.
Â
Introduction
Embryonic stem cells (ESCs) have the capacity to differentiate into all types of cells derived from the three germ layers Chambers and Smith, 2004 . The pluripotency of ESCs is what makes them attractive candidates for disease application. However, the application of ESCs for preclinical and clinical studies is not trivial since there are ethical concerns that must be considered Holm, 2015 . Reprogramming of differentiated cells using a viral-based method to deliver the reprogramming factors is a novel method to generate induced pluripotent stem cells (iPSCs) Carey et al., 2009 Takahashi and Yamanaka, 2006 Yu et al., 2007 . The potential of iPSCs is similar to that of ESCS in that iPSCs, too, have the capacity to differentiate into many types of cells from the three germ layers, as well as the ability to form teratomas and generate chimeras Bradley et al., 1984 Okita et al., 2007 . The preferential use of iPSCs can be a strategy that enables the generation of patient-specific stem cells while bypassing the ethical concerns associated with somatic cell nuclear transfer (SCNT) and human embryonic stem cells Stadtfeld and Hochedlinger, 2010 . This platform can be a promising tool for regenerative medicine applications and genetic disease models Amabile and Meissner, 2009 Hanna et al., 2007 Tateishi et al., 2008 Wernig et al., 2008 .
Our study aimed to examine the development of iPSCs via reprogramming of mouse embryonic fibroblasts (MEFs) through transduction with defined factors in polycistronic vector containing 4 pluripotent genes included Oct3/4, Sox2, Klf4 and c-Myc.
Materials-Methods
Isolation and culture of mouse embryonic fibroblasts and HEK 293T
Mouse embryonic fibroblasts (MEFs) were isolated from mouse embryos, according to a previously published protocol Jozefczuk et al., 2012 . Cells were cultured in DMEM/F12 complete (DMEM/F12, 10% FBS and 1% antibiotic; all reagents were bought from Life Technologies, Carlsbad, CA) until cells reached 80-90% confluency on surface flask. The MEFs were then sub-cultured to the 3rd-5th passages. Cells were cryopreserved in liquid nitrogen until use in experiments. HEK 293T cells were obtained from a commercial source (Life Technologies, Carlsbad, CA). The cells were thawed and cultured at 5 x 10 6 cells in a 25cm2 flask. HEK 293T cells were also cultured in DMEM/F12 complete.
Viral vector production
The viral vector was produced as previous published protocol Van Pham et al., 2016 . The viral vector used in this study encoded four factors: Oct3/4, Sox2, Klf4 and c-Myc (FUW-SOKM, (code 20325) Addgene, Cambridge, MA; this plasmid from Rudolf Jaenisch Laboratory). HEK 293T cells were trypsinized to obtain single cells. Then, the plasmid FUW-SOKM was co-transfected into HEK293T cells with pUMVC (code 8449) and pCMV-VSV-G (code 8454) (Addgene, Cambridge, MA) to produce a viral vector (FUW-SOKM vector). The mix was transferred into 2 mm electroporation cuvettes and transfected into cells. Immediately after, pre-warmed medium was gently added to the transfection mix and transferred into 6-well plates. The plates were incubated at 37oC, 5% CO2 for 24 h. After 36 h, supernatant was collected to extract viral particles by centrifugation.
Transduction into MEFs
On the day of transduction, MEFs were treated with polybrene at a final concentration of 8 μg/mL for 6 h, then transduced with viral vectors. Transduction was repeated three independent times, without polybrene treatment. The medium was refreshed after 2 d of transduction with induced pluripotent stem cell medium and every day until day 21. The iPSC medium was prepared by DMEM/F12 medium supplemented with KSR (Knockout serum replacement), 10 mM MEM Non-Essential Amino Acids Solution, 10 µg/mL bFGF, 4 ng/mL β-mercaptoethanol 1000X, 10ng/mL LIF (Leukemia inhibitor factor) (all chemicals and media were purchased from Life Technologies, Carlsbad, CA).
Culture of iPSCs
iPSCs were grown on MEF feeder layer that deactivated by mitomycin C in iPSC medium until cells reached 80-90% confluency on the surface of the 6-well plates. The cells were expanded by mechanical passaging.
Gene expression by reverse transcription real-time polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted with the use of easy-BLUE TM Total RNA Extraction Kit (iNtRON, Korea). Verification of iPSC gene expression was assessed via qPCR using qPCRBIO 1-Step SyGreen Detect and Go Kit (PCR Biosystems, England), according to the manufacturers’ protocol. The reaction was performed in a thermal real-time PCR cycler (Eppendorf, Germany) with reverse transcription at 45°C for 10 min (reverse transcription), 95°C for 2 min (denaturing) and amplification of 40 cycles at 95°C for 15 s (denaturing), 58°C for 15 s (annealing), and 72°C for 20 s (primer extension). The primers (AIT Biotech, Singapore) used in this experiment are listed in Table 1 (F: forward, R: reverse). RNA for GAPDH was co-amplified to assess the quality of the samples.
Table 1.
Flow cytometry
Cells were detached using trypsin/EDTA 0.25 % (Life Technologies), resuspended in PBS-containing 2 % FBS and 2 mM EDTA, and then stained with fluorochrome-labeled mAbs for 30 min at room temperature. Cells were washed using FACSFlow (BD Biosciences, San Jose, CA) and resuspended in FACSFlow (BD Biosciences). These cells analyzed SSEA-1, Vimentin and Thy-1 expression.
The MEFs were used as control. Cells were analyzed for cell surface marker expression using a FACSCalibur flow cytometer (BD Bioscience) and CellQuest Pro software (BD Biosciences).
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 30 min and subsequently washed two times with PBS. Cells were perforated through cell membranes using perforated membranous solution and washed again two times with PBS. Cells were incubated with Hoechst 33342 and primary antibodies (Santa Cruz Biotechnology) for 24 h in the dark at 40C, and washed with PBS. Lastly, cells were incubated with the appropriate fluorophore-labeled secondary antibodies (Santa Cruz Biotechnology) for 24 hours, dark, 4°C in FACSFluid. Specimens were analyzed on an Olympus fluorescence microscope (Carl-Zeiss, Germany) and images were acquired with a Zeiss Axiocam camera (Carl-Zeiss, Germany).
Alkaline Phosphatase (AP) enzymatic assay
Alkaline phosphatase (AP) enzymatic assay is detectable in the early stages of iPSC identification. AP activity is often used to distinguish iPSCs from feeder cells and parental cells in reprogramming experiments. Cells were incubated with AP (Life Technologies, Carlsbad, CA) for 30 min and analyzed on a fluorescence microscope. Images were acquired with a Zeiss Axiocam camera.
Teratoma formation assay
Approximately 10 3 -10 4 iPSCs were injected subcutaneously in the testicular region of Swiss mice, 4-6 weeks old. After 10 weeks, when teratomas formed, each teratoma was excised and fixed in 10% formalin overnight and later paraffin. The samples were sliced at 3-5ÎĽm thickness and stained with hematoxylin and eosin (H&E). The stained sections were then examined under a microscope.
Statistical analysis
Statistical analyses of all endpoints were performed using the two-sided Student’s t-test analysis of variance. All data are presented as mean ± SD. Statistical significance was considered at p < 0.05. Data were analyzed with Prism 6.0 software (GraphPad Software, La Jolla, CA).
Results
Culture and proliferation of MEFs and HEK293T cells
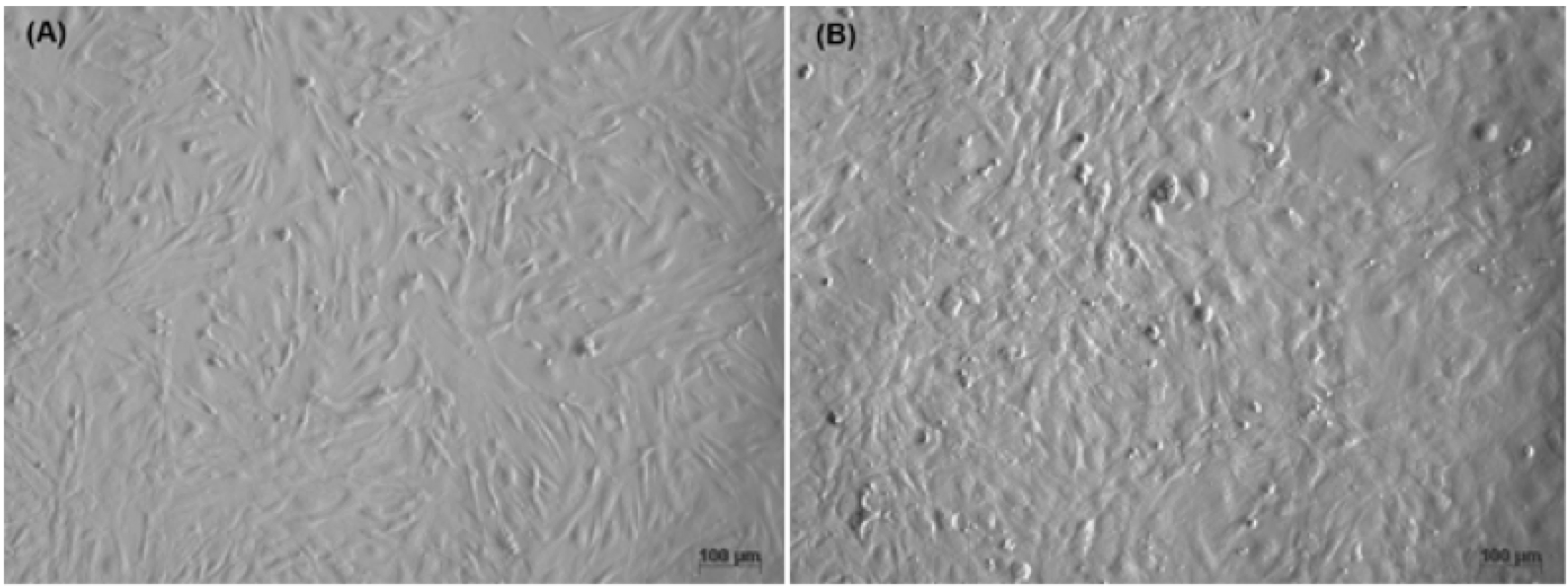
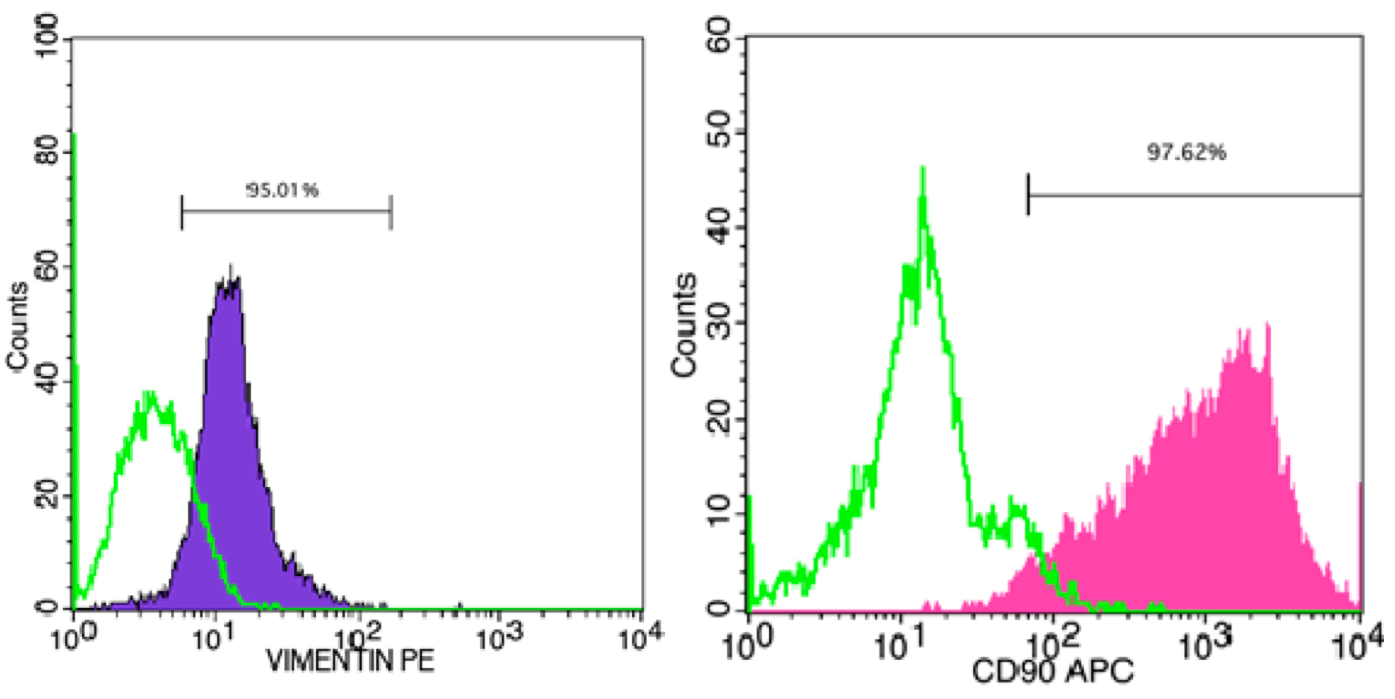
MEFs were isolated and cultured from mouse embryos, 12.5 days post coitum (dpc) – 13.5 dpc. After mouse embryos were incubated with trypsin/ EDTA-0.25%, a variety of cell types were dissociated, including fibroblasts, mesenchymal cells and epithelial cells ( Figure 1 ). The homogeneity of the cell population was confirmed by surface expression of CD90 (Thy-1) and Vimentin. Flow cytometric analysis of CD90 and Vimentin revealed that 97.6% and 95.0% fibroblasts expressed those genes, respectively ( Figure 2 ).
Figure 1. Primary culture MEFs
(A) Cells with mesangial, diamond, and small nuclei. (B) Cells with epithelial, pea, and large nuclei.
Figure 2. Flow cytometric detection of Thy-1 and Vimentin markers in MEFs
(A) Vimentin; (B) Thy-1
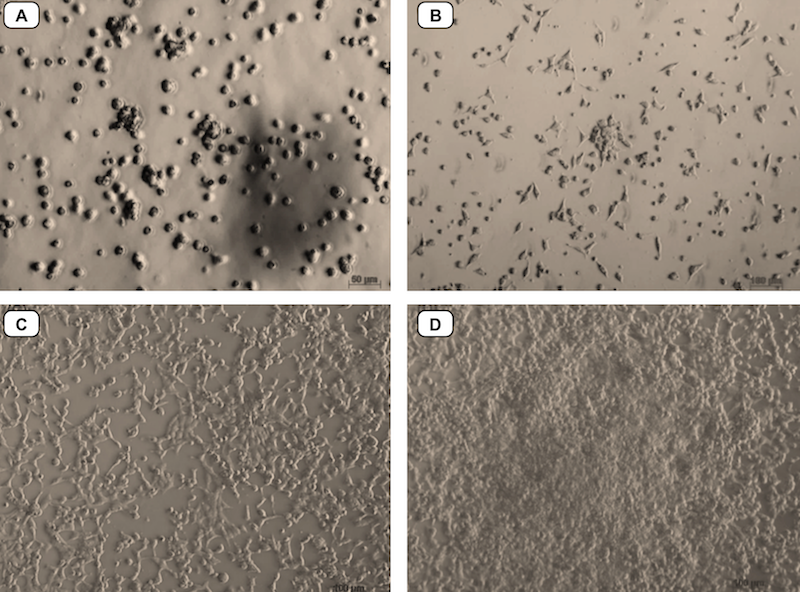
HEK293T cells were thawed from the commercial kit and cultured in standard conditions, according to the manufacturers’ guidelines. After thawing, the cells were cultured until they reached confluency (typically after 4 d). All cells were sub-cultured and frozen before use in studies ( Figure 3 ).
Figure 3. Culture and proliferation of HEK 293T cells
(A) Cells after thawing. (B) Cells after 24 h of culture. (C, D) Cells reach a homogenous population
Changes in cell morphology during reprogramming
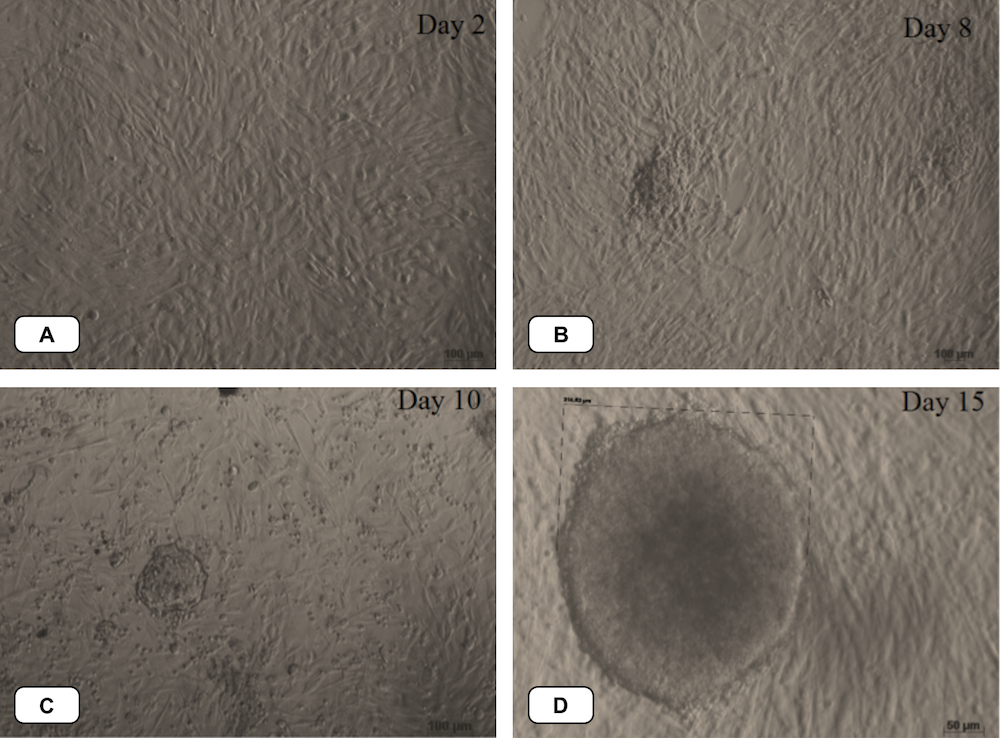
After transduction with a retroviral vector encoding the 4 factors, the MEFs were cultured in ES medium (which maintains ESC pluripotency). In addition, to increase the efficiency of viral transduction, the MEFs were infected by viral vector four times. Under this condition, almost all the cells died and the remaining surviving cells changed morphology from mesenchymal cell type to rounded, squamous epithelial cell type at about day 8. The cells formed clusters around days 15-21. The small clumps were clearly distinguishable from the MEF feeder cells. Some of the cell clusters displayed the typical ESC morphology ( Figure 4 ). There were approximately 10 – 20 ESC-like clusters, with about 10 5 in cell number and with size ranging from 300-500 µm. The reprogramming efficiency was about 0.01-0.02%.
Figure 4. Transduced MEFs change their shape from mesenchymal to epithelial
After transduction, cells changed their morphology from mesenchymal cell type (A) to rounded, squamous epithelial cell type around day 8 (B), day 10 (C). The cells formed clusters around days 15 (D)
Up-regulation of pluripotent markers
The clusters of cells with ESC morphology were sub-cultured for several passages in ES medium. The cells expressed markers of pluripotency as evaluated by flow cytometry and immunocytochemistry. The percentage of SSEA-1 positive cells was 8.58 ± 0.03% (p>0.05) in transduced cells and 0% in the control.
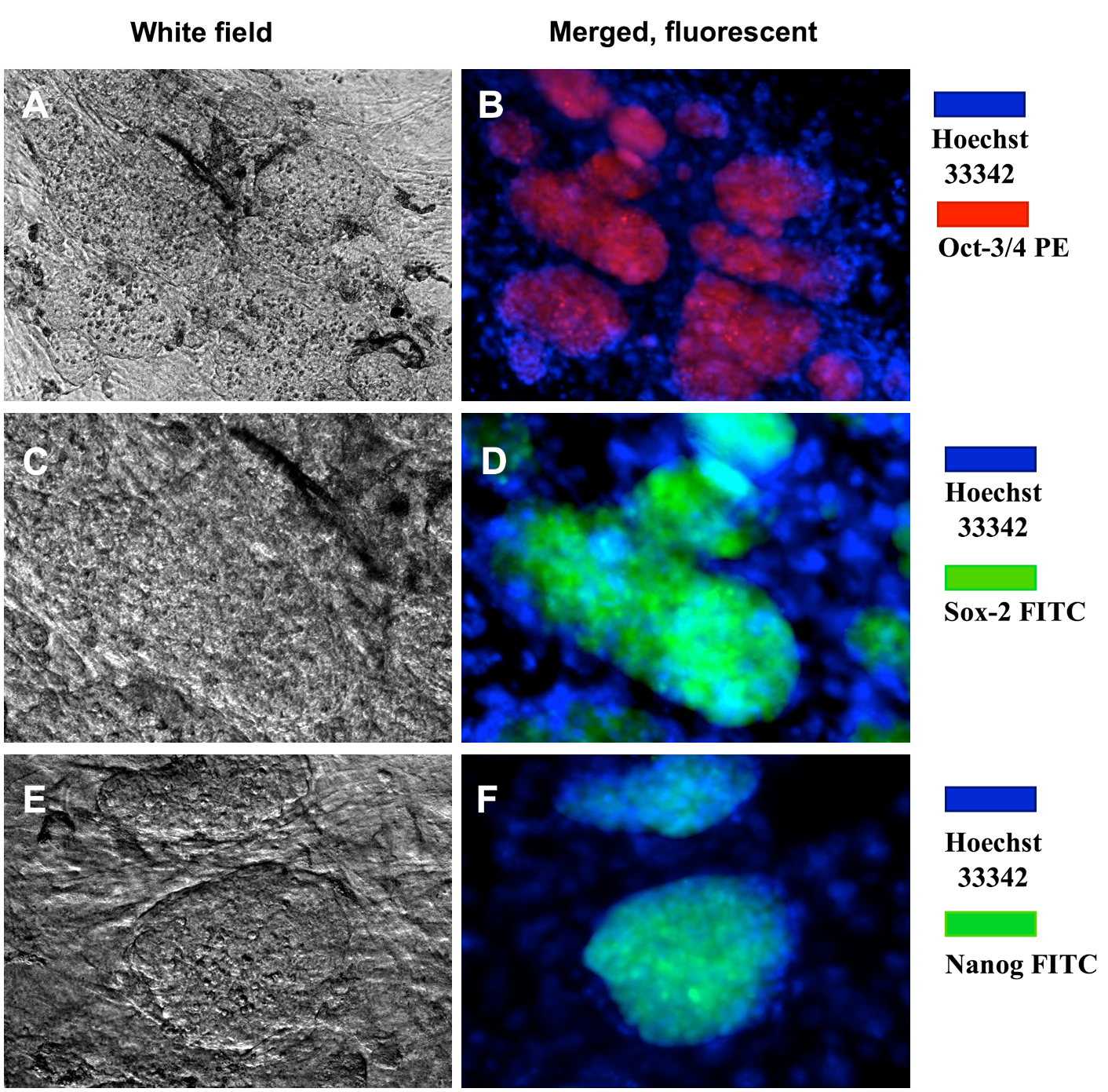
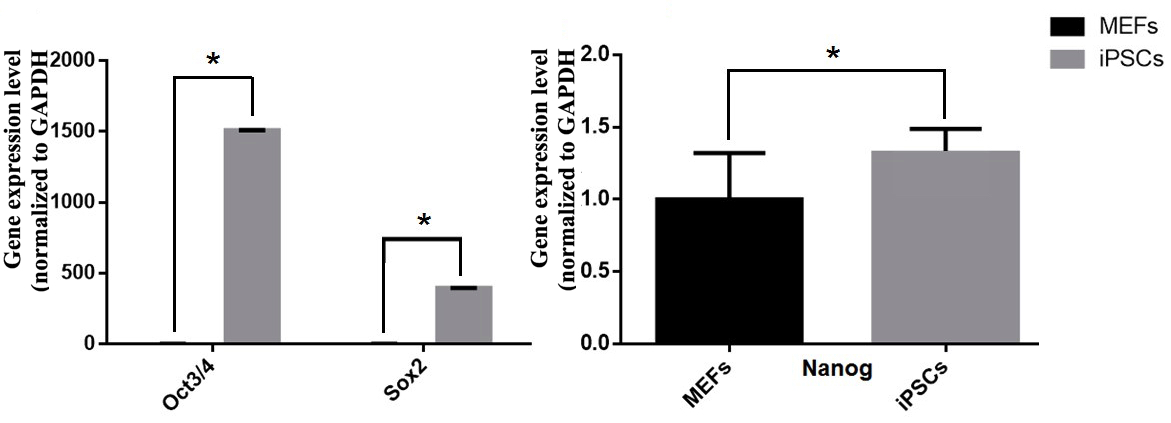
The expression of pluripotent markers (e.g. Oct4, Sox2 and Nanog) was evaluated by immunocytochemistry ( Figure 5 ). The results showed that the clumps (clusters) of transduced cells were positive for Oct4, Sox2 and Nanog markers; the MEF feeder cells did not express these markers. Moreover, markers of pluripotency were evaluated by RT-qPCR. Primers were amplified only for the endogenous oct4 (oct4-endo) and sox2 (sox2-endo) to discriminate from the transgene-encoded transcripts. The RT-qPCR results revealed that transduced cells had an increased expression of pluripotent genes, such as oct4, sox2 and nanog ( Figure 6 ); indeed, the expression of those genes in reprogrammed cells were 1509.65, 395.26 and 1.32 fold higher, respectively, than in those
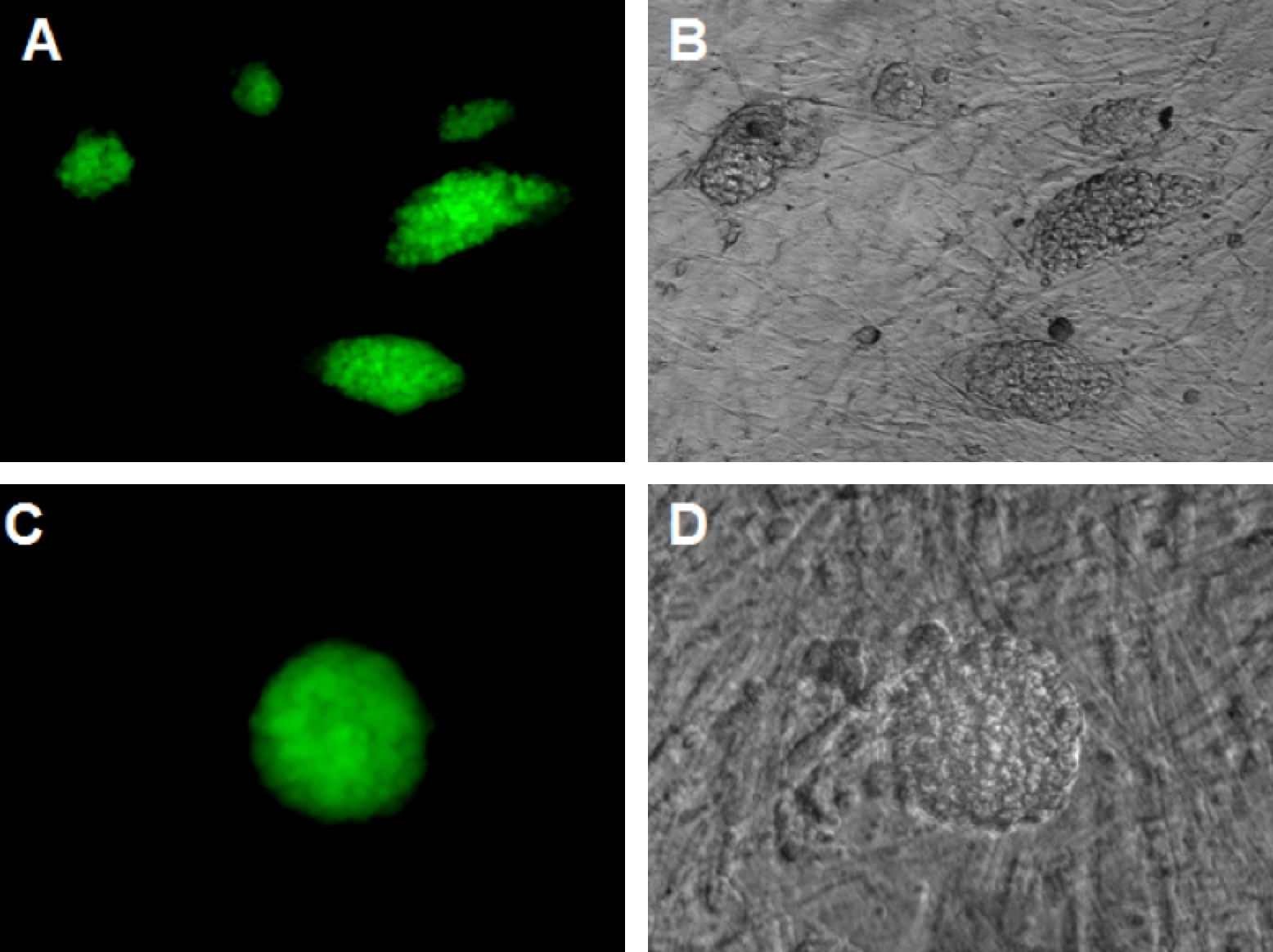
Figure 5. Immunochemistry of ES-like cell colonies expressing Oct4, Sox2 and Nanog markers
These colonies expressed Oct4 (A,B), Sox2 (C,D), and Nanog (E,F). Magnification X20
Figure 6. Expression of the pluripotent genes oct4-endo, sox2-endo and nanog in transgenic (transduced) cells compared to non-transgenic (non-transduced) cells
(A) oct4-endo; sox2-endo. (B) nanog. (*: P <0.05).
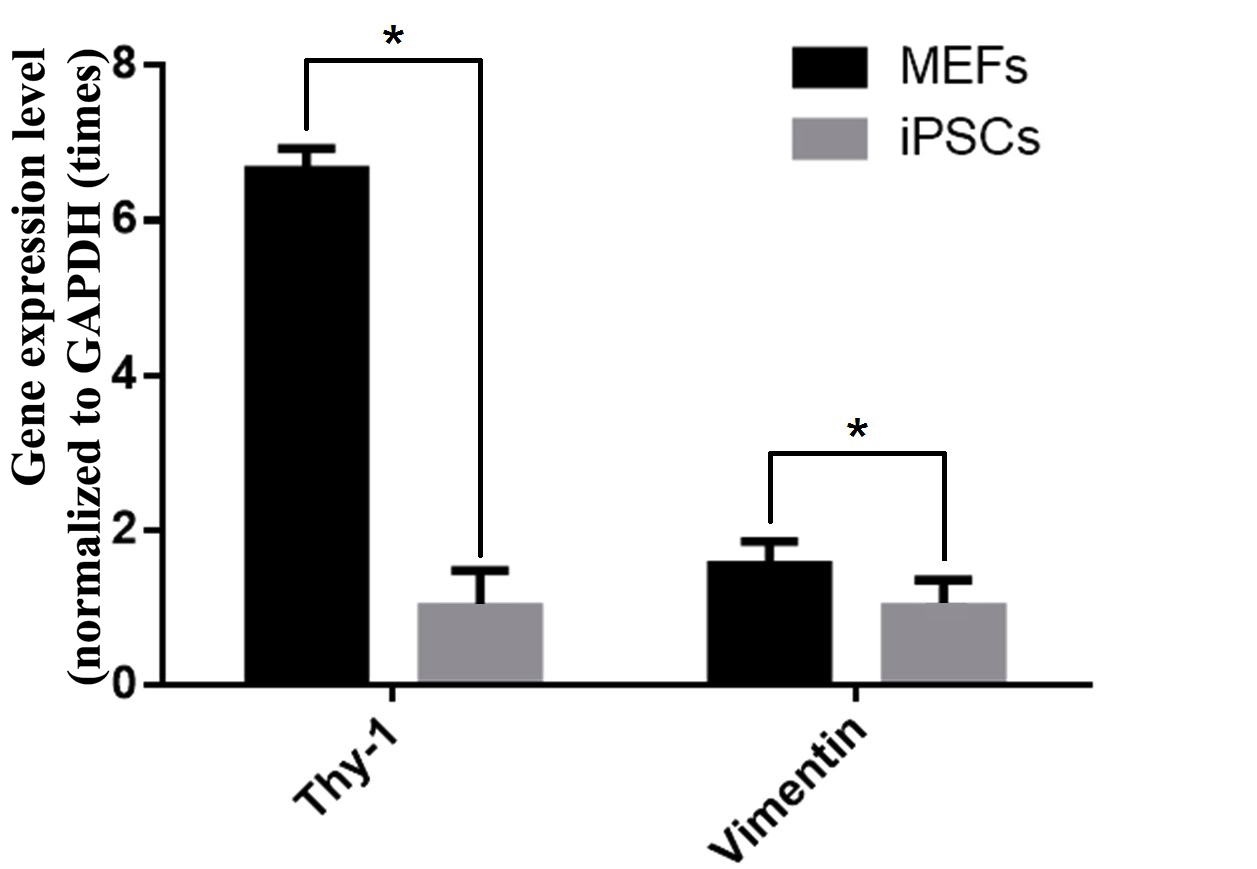
cells without reprogramming. Moreover, RT-qPCR also revealed decreased expression of MEF genes (vimentin and thy-1) ( Figure 7 ) in transduced cells compared to MEF cells. In particular, the typical MEF-related genes (such as thy-1 and vimentin) in non-transduced cells were expressed at 6.65 and 1.55 fold higher levels than in transduced cells.
Figure 7. Expression of the pluripotent genes thy-1 and vimentin in transgenic cells versus non-transgenic cells
(*: p <0.05)
Alkaline phosphatase (AP) assay
The ESC-like colonies were picked placed onto a MEF feeder layer, and then analyzed for AP activity. The results showed that the ESC-like colonies expressed high activity of AP ( Figure 8 ), in contrast to MEFs without viral vector transduction.
Figure 8. AP activity of ES morphological clusters
The colonies were positive AP, while the MEFs were negative. (A, B): magnification X10, (C, D): magnification X20
In vivo teratoma formation
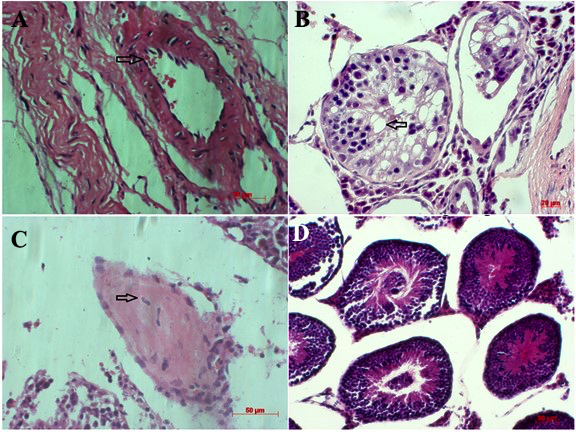
To evaluate the ability of the ESC-like clusters to form teratomas in vivo, colonies having ESC-like morphology were injected into mouse testicles at a density of 10 3 -10 4 cells. Histological analyses demonstrated that those cells induced teratoma formation and contained differentiated cells derived from all three germ layers ( Figure 9 ). Specifically, keratinocytes and neonatal crest-like structures originated from epidermal, while skeletal muscle, adipose and cartilage-like structures were derived from mesoderm. Moreover, epidermal and intestinal epithelial-like structures (originating from endoderm) were also observed.
Figure 9. Formation of teratomas from ESC-like cell clusters
Histological evaluation of the ESC-like cell clusters indicated that these cells induced teratomas which contain some differentiated cells derived from all three germ layers (A, B, C and D)
Discussion
ESCs are advantageous for regenerative medicine applications given their pluripotency feature, i.e. their ability to differentiate into a variety of cell types derived from the three germ layers. However, ESCs have not been widely used due mainly to ethical concerns. In 2012, the Nobel Medicine Prize was awarded to John B. Gurdon and Shinya Yamanaka for their works that re-established cellular programming in differentiated cells as a means to create and maintain a pluripotent state Takahashi et al., 2007 Takahashi and Yamanaka, 2006 Yu et al., 2007 . Such cells are called induced pluripotent stem cells (iPSCs); they have many ESC-like characteristics. These potent iPSCs are essential cell sources for regenerative medicine and tissue engineering. Our study herein was aimed at reprogramming fibroblasts to generate into iPSCs via the use of a viral-based delivery of reprogramming factors (e.g. Oct3/4, Sox2, Klf4 and c-Myc) in ES medium.
Fibroblasts were isolated from mouse embryos. The cell population was a homogenous population (97.6 % of the population expressed CD90 and about 95% of the population expressed Vimentin). Indeed, CD90 and Vimentin expression are considered to be markers of fibroblasts Saalbach et al., 1996 Strutz et al., 1995 . Fibroblasts are the most common cells of connective tissue in animals that have important roles in synthesizing the ECM and wound healing. These cells are also conveniently isolated and vigorously proliferated. As a result, fibroblasts are one of the most popular cell candidates for stem cell research studies as well as in application of reprogramming.
Interesting, fibroblasts were first used, by Yamanaka et al., to reprogram into iPSCs Takahashi and Yamanaka, 2006 . After that, the potent cells can be converted into cardiomyocytes Efe et al., 2011 , functional neurons Vierbuchen et al., 2010 , multilineage blood progenitors Szabo et al., 2010 , hepatocytes Sekiya and Suzuki, 2011 and endothelial progenitor cells Margariti et al., 2012 .
In this study, fibroblasts from mouse embryos (MEFs) were reprogrammed to iPSCs through transduction with a single polycistronic viral vector encoding for mouse cDNAs of Oct3/4, Sox2, Klf4 and c-Myc.
Our study investigated a strategy that was similar to the one by Yamanaka et al. Takahashi and Yamanaka, 2006 . The results showed that the reprogramming efficiency was about 0.01-0.02%, which is approximately that of previously published studies Takahashi and Yamanaka, 2006 . Our data demonstrates that the fibroblast culture method, viral vector production and reprogramming technology were all effective. The percentage of SSEA-1 positive cells was significantly lower than previous experiments. However, immunochemistry analyses revealed that cells with the highest ESC-like morphological (those in clumps/clusters) strongly expressed pluripotent markers (Oct4, Sox2 and Nanog).
It is possible that the lower percentage of SSEA-1 positive cells is a consequence of the quantity of cells evaluated. For example, for flow cytometry, the cell number assayed was quite small (about 103 cells). Moreover, it is difficult to isolate ESC-like clumps from the MEF feeder. Thus, the ratio of SSEA-1 positive cells was expected to decrease. Finally, ESC-like clusters can be differentiated during the culture process. Together with flow cytometry and immunochemistry results, the RT-qPCR results showed that oct4 gene was strongly expressed in transduced cells, but barely expressed in MEFs (non-transduced). The Oct4 gene is used to identify ESCs and the level of oct4 gene expression is related to the pluripotency of the cells Na et al., 2010 .
The transduced cells also greatly expressed other pluripotent genes, such as sox2 and nanog. In many previous studies, a variety of specific genes in differentiated cells were down-regulated or “silenced” during reprogramming to form and maintain a pluripotent state Buganim et al., 2013 . In this study, genes thy-1 and vimentin were evaluated in transduced cells. The results showed that ESC-like clusters showed a down-regulation of those genes, compared to non-transduced cells; these results are somewhat similar to findings from past research studies Liu et al., 2012 Trevisan et al., 2017 .
To investigate the pluripotent characteristics of transduced cells, ESC-like cell clumps were test using the AP assay. AP activity is frequently used to distinguish stem cells from feeder cells as well as from parental cells in reprogramming Stefkova et al., 2015 . The results showed that ES-like colonies expressed high activity of AP, in contrast to MEF feeder cells which show no AP activity. Moreover, activity of AP in cells in the inner portion of the colonies was greater than that of cells in the outer portion. Despite co-culturing with MEF feeders, iPSCs were easily differentiated so that low or no AP activity was detected Lu et al., 2011 . In addition, ESC-like cell clusters were evaluated for their ability of form teratomas in vivo. Data from our study reveal that the potent cells could be differentiated into many types of cells derived from the three embryonic germ layers, which was similar to conclusions from previous studies Carey et al., 2009 Ross et al., 2010 .
Conclusion
In this study, we generated mouse iPSCs from MEFs using a polycistronic viral vector encoding mouse cDNAs for Oct3/4, Sox2, Klf4 and c-Myc. Using this approach, we showed that the transduced cells changed their morphology, formed ESC-like cell clusters, clearly distinguishable from the MEF feeder. Moreover, these potent cells showed strong expression of pluripotent markers including SSEA-1, Oct3/4, Sox2 and Nanog, strong AP activity and also formed teratomas in vivo. Overall, this study demonstrates that fibroblasts can be reprogrammed into iPSCs. This technology can greatly benefit regenerative medicine applications and genetic disease models.
Abbreviations
AP: Alkaline phosphatase
ESCs: Embryonic Stem Cells
HEK 293T: Human embryonic kidney cells 293T
iPSCs: Induced Pluripotent Stem Cells
MEFs: Mouse Embryo Fibroblasts
Oct4: Octamer-binding transcription factor 4
Sox2: Sex determining region Y-box 2
SSEA-1: Stage Specific Embryonic Antigen-1.
Author Contribution
OTH was responsible for creating the experiment design, data analysis, preparing the figures, performing the MEFs, HEK 293T and iPSCs cultures, transducing viral vector into MEFs, teratoma assay, and writing the Results, Discussion and Conclusion. MT-HT was responsible for producing the viral virus vector, performing RT-PCR and RT-qPCR analysis and writing the Introduction and Materials-Methods PVP was responsible for performing the essays of Flow cytometry, Immunocytochemistry, AP assay, revising the manuscript. All authors read and approved the manuscript.
References
- G. Amabile, A. Meissner. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends in molecular medicine. 2009;15:59-68. Google Scholar
- A. Bradley, M. Evans, M.H. Kaufman, E. Robertson. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255-256. Google Scholar
- Y. Buganim, D.A. Faddah, R. Jaenisch. Mechanisms and models of somatic cell reprogramming. Nature reviews Genetics. 2013;14:427-439. Google Scholar
- B.W. Carey, S. Markoulaki, J. Hanna, K. Saha, Q. Gao, M. Mitalipova, R. Jaenisch. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:157-162. Google Scholar
- I. Chambers, A. Smith. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150-7160. Google Scholar
- J.A. Efe, S. Hilcove, J. Kim, H. Zhou, K. Ouyang, G. Wang, J. Chen, S. Ding. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nature cell biology. 2011;13:215-222. Google Scholar
- J. Hanna, M. Wernig, S. Markoulaki, C.W. Sun, A. Meissner, J.P. Cassady, C. Beard, T. Brambrink, L.C. Wu, T.M. Townes. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920-1923. Google Scholar
- S. Holm. Biobanking human embryonic stem cell lines: policy, ethics and efficiency. Monash bioethics review. 2015;33:265-276. Google Scholar
- J. Jozefczuk, K. Drews, J. Adjaye. Preparation of Mouse Embryonic Fibroblast Cells Suitable for Culturing Human Embryonic and Induced Pluripotent Stem Cells. Journal of Visualized Experiments : JoVE. 2012;3854:. Google Scholar
- Y. Liu, D. Cheng, Z. Li, X. Gao, H. Wang. The gene expression profiles of induced pluripotent stem cells (iPSCs) generated by a non-integrating method are more similar to embryonic stem cells than those of iPSCs generated by an integrating method. Genetics and molecular biology. 2012;35:693-700. Google Scholar
- H.E. Lu, M.S. Tsai, Y.C. Yang, C.C. Yuan, T.H. Wang, X.Z. Lin, C.P. Tseng, S.M. Hwang. Selection of alkaline phosphatase-positive induced pluripotent stem cells from human amniotic fluid-derived cells by feeder-free system. Experimental cell research. 2011;317:1895-1903. Google Scholar
- A. Margariti, B. Winkler, E. Karamariti, A. Zampetaki, T.N. Tsai, D. Baban, J. Ragoussis, Y. Huang, J.D. Han, L. Zeng. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13793-13798. Google Scholar
- J. Na, J. Plews, J. Li, P. Wongtrakoongate, T. Tuuri, A. Feki, P.W. Andrews, C. Unger. Molecular mechanisms of pluripotency and reprogramming. Stem cell research & therapy. 2010;1:33. Google Scholar
- K. Okita, T. Ichisaka, S. Yamanaka. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313-317. Google Scholar
- P.J. Ross, S.T. Suhr, R.M. Rodriguez, E.A. Chang, K. Wang, K. Siripattarapravat, T. Ko, J.B. Cibelli. Human-induced pluripotent stem cells produced under xeno-free conditions. Stem cells and development. 2010;19:1221-1229. Google Scholar
- A. Saalbach, U. Aneregg, M. Bruns, E. Schnabel, K. Herrmann, U.F. Haustein. Novel fibroblast-specific monoclonal antibodies: properties and specificities. The Journal of investigative dermatology. 1996;106:1314-1319. Google Scholar
- S. Sekiya, A. Suzuki. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390-393. Google Scholar
- M. Stadtfeld, K. Hochedlinger. Induced pluripotency: history, mechanisms, and applications. Genes & development. 2010;24:2239-2263. Google Scholar
- K. Stefkova, J. Prochazkova, J. Pachernik. Alkaline phosphatase in stem cells. Stem cells international. 2015;2015:628368. Google Scholar
- F. Strutz, H. Okada, C.W. Lo, T. Danoff, R.L. Carone, J.E. Tomaszewski, E.G. Neilson. Identification and characterization of a fibroblast marker: FSP1. The Journal of cell biology. 1995;130:393-405. Google Scholar
- E. Szabo, S. Rampalli, R.M. Risueno, A. Schnerch, R. Mitchell, A. Fiebig-Comyn, M. Levadoux-Martin, M. Bhatia. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521-526. Google Scholar
- K. Takahashi, K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda, S. Yamanaka. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. Google Scholar
- K. Takahashi, S. Yamanaka. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. Google Scholar
- K. Tateishi, J. He, O. Taranova, G. Liang, A.C. D'Alessio, Y. Zhang. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. The Journal of biological chemistry. 2008;283:31601-31607. Google Scholar
- M. Trevisan, G. Desole, G. Costanzi, E. Lavezzo, G. Palu, L. Barzon. Reprogramming Methods Do Not Affect Gene Expression Profile of Human Induced Pluripotent Stem Cells. International journal of molecular sciences. 2017;18:. Google Scholar
- P. Van Pham, N.B. Vu, M.T.-H. Truong, O.T. Huynh, H.T. Nguyen, H.L. Pham, N.K. Phan. Hepatocyte growth factor improves direct reprogramming of fibroblasts towards endothelial progenitor cells via ETV2 transduction. Biomedical Research and Therapy. 2016;3:45. Google Scholar
- T. Vierbuchen, A. Ostermeier, Z.P. Pang, Y. Kokubu, T.C. Sudhof, M. Wernig. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035-1041. Google Scholar
- M. Wernig, J.P. Zhao, J. Pruszak, E. Hedlund, D. Fu, F. Soldner, V. Broccoli, M. Constantine-Paton, O. Isacson, R. Jaenisch. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5856-5861. Google Scholar
- J. Yu, M.A. Vodyanik, K. Smuga-Otto, J. Antosiewicz-Bourget, J.L. Frane, S. Tian, J. Nie, G.A. Jonsdottir, V. Ruotti, R. Stewart. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. Google Scholar
 Biomedpress
Biomedpress
 Open Access
Open Access 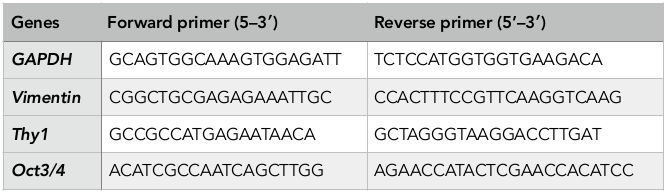







Primer sequences of iPSC genes in RT-qPCR analysis