Abstract
Introduction: Type 1 diabetes mellitus (T1D) disease is caused by lesions or dysfunction of beta cells of pancreatic islets, causing less insulin to be secreted into the blood and thereby increasing glucose levels in the blood. In this study, we evaluated and compared the efficiency of treatment for T1D using autograft and allograft adipose-derived stem cells (ADSCs). Methods: ADSCs were collected from the belly of mice before they were injected using a single dose of streptozotocin (100 mg/kg) to induce T1D. T1D mice were intravenously injected with a dose of 2x106 ADSCs into the tail vein. Therapeutic efficacy was assessed by survival rate, blood glucose levels, serum insulin levels, histology and immunohistochemistry of pancreatic islets. Results: The results showed that both autograft and allograft transplantation of ADSCs demonstrated similarities in mortality rate, blood glucose level, blood insulin level, quantity and size of pancreatic islets. Both transplantations significantly improved T1D mice, which showed a decrease in mortality rate as well as blood glucose level, and increases in blood insulin level, quantity and size of pancreatic islets. Conclusion: The similar results suggest that both autologous and allogeneic transplantations of ADSCs are promising therapy for T1D treatment.        Â
Introduction
Diabetes mellitus disease is caused by lesions or dysfunction of beta cells in pancreatic islets, resulting in inadequate production and usage of insulin leading to abnormally high blood glucose levels. There are over 422 million diabetic patients in the world today and this number is expected to double by 2025 Halban et al., 2014 Organization, 2016 . Diabetes mellitus is a chronic disease with many complex symptoms related to blood glucose rise and associated ketoacidosis; such symptoms include slow healing cuts, blurred vision, numbness in feet and legs, and nausea and vomiting. There are two main kinds of diabetes mellitus: diabetes type 1 and type 2. The main general characteristic of both types is extremely high blood glucose level which cannot be controlled. Worldwide there are about 16 million type 1 diabetic patients, about 5% - 10% of all diabetic cases, mainly in children and adolescent Halban et al., 2014 Organization, 2016 .
Diabetes mellitus type 1 (T1D) patients often manifest other physiological characteristics, such as increased appetite and thirst, increased urination and weight loss Organization, 2016 . Although T1D can be treated by supplementing insulin into the body, a major disadvantage of this approach is the patients completely depend on exogenous insulin. The long-term usage of insulin may control blood glucose levels less and may cause lipodystrophy and other serious complications, such as retinopathy, blindness, cardiovascular disorders, stroke, kidney failure and cerebrovascular diseases.
Pancreas/islet transplantation has been one alternative therapy for diabetes treatment but currently stem cell transplantation has garnered wider interest. Pancreas/islet transplantation is challenging due to limited tissue source which impedes it from being a widespread application. New investigations into development of new therapies, such as stem cell therapy, for diabetes treatment have to led to promising preclinical research results; stem cell transplantation have shown efficiency for diabetes treatment Karaoz et al., 2013 Katuchova et al., 2015 Kwon et al., 2008 Lin et al., 2009 Yaochite et al., 2015 .
Stem cells from fat tissue is an abundant source of mesenchymal stem cells (MSCs). Among the different kinds of MSCs, adipose derived stem cells (ADSCs) are a great stem cell candidate due to its abundant supply and resource. In fact, the proliferation of ADSCs has been demonstrated to be better than cells from bone marrow tissue. Several research studies have demonstrated ADSCs are able to differentiate into multiple cell lines, including adipocytes, osteoblasts, chondrocytes and even insulin-producing cells of pancreatic islets Bunnell et al., 2008 Karaoz et al., 2013 . Moreover, ADSCs also secrete cytokines which are involved in immune modulation and which support metabolic regulation in the body. Due to these properties, ADSCs are considered as a promising candidate for stem cell transplantation for treating T1D Lin et al., 2009 Lin et al., 2015 Paek et al., 2014 Zhou et al., 2016 Zuk, 2013 .
Herein, we evaluated the use of ADSC transplantation to treat T1D; we investigated both autologous and allogeneic ADSCs. We hypothesized that the effects of autologous and allogeneic transplantations of ADSCs might be similar; both might be efficacious given the immune modulation property of ADSCs.
Materials-Methods
Cell isolation and proliferation
Murine ADSCs were isolated and proliferated according to a previously published protocol Vu et al., 2015 . Briefly, fat tissues were obtained from mice genital fat. Cell isolation was performed using ADSC Extraction kit (GeneWorld Ltd., Vietnam). After collection, cells were cultured in MSCCult medium (RegenMed Ltd., Ho Chi Minh, Vietnam) containing DMEM/F12 supplemented with 10 ng/mL epidermal growth factor (EGF), 10 ng/mL basic fibroblast growth factor (bFGF) and 10% fetal bovine serum (FBS), at 370C and 5% CO2, and refreshed every 3 days; except for media all regents were purchased from Sigma-Aldrich (St. Louis, MO).
In vitro differentiation
ADSCs were induced to differentiate into adipogenic cells and osteogenic cells in specific differentiation medium. For adipogenic differentiation, cells were plated and cultured in DMEM/F12 supplemented with 0.5 μM dexamethasone, 50 μM indomethacin, 1.5 μg/mL insulin, and 0.5 μM 3-isobutyl-1-methylxanthine (IBMX) (all reagents were purchased from Sigma-Aldrich). After 5-7 days, the intracellular lipid accumulations were evaluated by Oil red O staining (Sigma-Aldrich, St Louis, MO). For osteogenic differentiation, cells were plated and cultured in DMEM/F12 medium supplemented with 10% FBS, 1% antibiotic-antimycotic 100X, 100 nM dexamethasone, 10 mM beta-glycerol, 10 μM ascorbic acid, 20.8 mg/mL L-leucine, and 100 ng/mL L-lysine (all purchased from Sigma-Aldrich). After 14-21 days, calcium accumulation in the extracellular matrix was confirmed by Alizarin Red staining (Sigma-Aldrich, St Louis, MO).
Inducing T1D mouse model by streptozotocin
Mice weighing 25-28 g were selected and stably farmed for 2 weeks before induction of diabetes. All procedures and manipulations were performed in accordance with instructions and approval of the Animal Care Committee. The mice were fasted for 24 hours before streptozotocin (STZ) injection. STZ (purchased from Santa Cruz Biotechnology, Dallas, TX) was dissolved in cooled 0.01 M citrate buffer at pH 4.5. The T1D mice were induced by a single intraperitoneal injection of 100 mg/kg of STZ. Mouse blood glucose was examined by Accu-Chek Active (Mannheim, Germany). Unlike T1D mice which received STZ injection, control (normal) mice received PBS.
Mouse ADSC transplantation
After 3 weeks, STZ-induced diabetic mice were intravenously injected with 2x106 mouse ADSCs, either autologous or allogeniec, in 200 μL sterile phosphate buffered saline (PBS; Sigma-Aldrich) per mouse. The treated mice were monitored for survival rate, blood glucose levels, serum insulin and morphological analysis of pancreatic islets. The placebo treatment was injection with PBS only.
Glucose and insulin tolerance tests
Glucose tolerance tests were conducted on the mice at 8 weeks after the transplantation. Mice were fasted 6-8 h before the glucose tolerance test. D-glucose was intraperitoneally injected into mice (1.5 g/kg body weight) and blood glucose levels were measured at 0, 15, 30, 60 and 120 minutes. Insulin was dissolved in PBS at a dose of 0.75 mg/kg body weight. Blood glucose levels of mice were measured at 0, 15, 30, 60 and 120 minutes after insulin treatment.
Serum insulin measurement
At day 56, mouse blood was obtained from facial vein and serum was collected by centrifugation at 3000 rpm in 10 minutes at 4oC. The transparent faint yellow serum was assessed to measure insulin concentrations by Mercodia Ultrasensitive Mouse Insulin Enzyme linked immunosorbent assay (ELISA) (Uppsala, Sweden). The calibrator curve was constructed and insulin concentrations from the samples were extrapolated from the standard curve (analysis by Prism 6, GraphPad Software, San Diego, CA).
Histological analysis of pancreatic islets
After 56 days of treatment, pancreases from mice were collected and fixed in 4% paraformaldehyde, treated with 30% sucrose overnight and embedded in optimal cutting temperature compound (OCT; Sigma-Aldrich). The 7 µm-thin sections were stained with Hematoxylin & Eosin (Sigma-Aldrich) following the standard established procedure. The pancreatic islets were observed by microscopy and were analyzed, counted and measured by Axio Vision Microscopy Software (Carl-Zeiss, Germany). For immunofluorescence staining, the 7 µm-thin sections were dipped in 1% bovine serum albumin (BSA; Sigma-Aldrich) for 1 hour at room temperature. The blocked slides were incubated with insulin antibody (Goat anti-Rabbit Alexa Fluor 488, Sigma-Aldrich) overnight at 40C, then stained with FITC-conjugated secondary antibody (sc-2012, Santa Cruz Biotechnology) for 2 hours. Finally, the sections were stained with Hoechst 33442 (Sigma-Aldrich) for nucleus staining and observed by fluorescence microscopy (Cell Observer, Carl-Zeiss, Germany).
Data analysis
The data were analyzed for statistical significance using GraphPad Prism software. Data were presented as mean ± SD. When applicable, a Student's unpaired t-test and one-way ANOVA were used to determine significance; p<0.05 was considered to be statistically significant.
Results
Isolation and characterization of adipose derived stem cells
Genital fat of mice was collected according to the protocol of the ADSC Extraction kit. Mononuclear candidates exhibited a round shape (red arrow, Figure 1A ) and were a multiple mix of red blood cells and platelets. Red blood cells were less adhesive and discarded when refreshing culture media. After 24 h, mononuclear cells began to attach and spread along the culture surface. At day 3, all unattached cells and red blood cells were discarded ( Figure 1B ) Vu et al., 2015 .
Figure 1.
After 5 d, cells began to adhere and proliferate to about 30 – 50% of cultured areas. Cell morphology was not homologous; most cells had an elongated, fibroblast-like shape while some were shorter and flat, and some had a comma shape ( Figure 1C ). From days 7-9, cell density reached about 70 – 80% confluence and cells could be sub-cultured for the 1st passage ( Figure 1D ). The sub-culture procedure allows for more space and nutrition for cell proliferation; through each sub-culture non-ADSCs were removed to enable the ADSC population to become more homogenous.
Cell morphology changed when ADSCs were cultured in ‘differentiation’ medium (i.e. explain again what the medium was). After 7 -12 d in this medium, cells differentiated into adipocytes with lipid droplet formation and showed positive staining with Oil red O ( Figure 2 ). Similarly, for osteoblast differentiation, after 14 d ADSCs changed their shape, accumulated intracellular calcium (Ca2+) and magnesium (Mg2+), and showed positive staining with Alizarin Red ( Figure 2 ). ADSC candidates were capable of differentiating into adipocytes and osteoblasts in vitro. These results are similar to previous publications Timper et al., 2006 Vu et al., 2015 .
Figure 2. Cell morphology of mADSCs
(A) Osteogenic cells on day 14, (B) staining of osteoblasts with Alizarin Red, (C) adipogenic cells on day 7, and (D) staining of adipocytes with Oil Red O.
ADSC transplantation reduced the mortality rate of diabetic mice
At 56 d after transplantation, except for the T1D-Placebo group, all other groups showed no mortality. In the control (normal) mouse groups which received autograft (autologous) or allograft (allogeneic) ADSC transplantation, the survival rate was 100%. Mice in these groups remained healthy, agile and active. These results showed that cell transplantation was safe for mice. Immediately after transplantation, mice did not get shocked or tired nor did they exhibit abnormal manifestation for the 56 days of follow-up. There were no differences between Normal-autograft group, Normal-allograft group and Normal-placebo group (mice which received placebo treatment) ( Figure 3 ).
Figure 3.
Kaplan–Meier survival curve comparing between the groups after mADSC transplantation
In T1D-placebo group, the health of the mice worsened and most mice became tired, lazy and unmotivated; only about 60% of mice survived at day 56 post transplantation. Due to the impact of STZ, the beta cells of pancreatic islets were destroyed, resulting in a high blood glucose level. Mice lost weight and exhibited multiple complications, such as corneal lesions, shuddering, and excitability. However, after cell transplantation, for both the T1D-autograft and T1D-allograft groups, the diabetic mice showed improvement of their health status; these included weight gain, smooth fur, and increased agility and activity. There were no mortality cases post cell transplantation, suggesting that stemcell transplantation could help prolong the life of T1D mice.
ADSC transplantation reduced blood glucose level of diabetic mice
In the control (normal) mouse groups, the blood glucose level remained stable during the 56 d follow-up after treatment. There was no statistical difference between Normal-autograft group, Normal-allograft group or Normal-placebo group. The results showed that ADSC treatment (autograft or allograft), like placebo treatment, did not cause hyperglycemia in normal (non-diabetic) mice. However, in T1D diabetic mice, ADSCs affected the regulation of mouse blood glucose levels; the blood glucose levels of the autograft and allograft transplanted groups strongly decreased from day 7 to 28. At day 56 after transplantation, the blood glucose levels of the autograft and allograft groups were 262±48 mg/dL and 233±36 mg/dL, respectively, which was maintained from day 28 to day 56 ( Figure 4 ).
Figure 4.
Comparison of blood glucose levels (mg/dL) between groups after ADSC transplantation
Glucose and insulin tolerance tests
After D-glucose injection, all mice exhibited hyperglycemia. In T1D-Placebo group, blood glucose metabolism was slowed down such that after 120 minutes, blood glucose levels did not return as pre-tolerance. Blood glucose levels of the remaining groups recovered after 120 minutes ( Figure 5 ). These results show that the treatment groups (T1D-Autograft and T1D-Allograft) have the ability to regulate blood glucose.
For insulin tolerance, serum insulin levels of all groups were reduced after insulin injection. Mice responded fairly to exogenous insulin and did not occur insulin-resistance after cell transplantation ( Figure 5B ).
Histological analysis of pancreatic islets and serum insulin measurements
After treatment, the pancreatic islets of T1D mice were recovered. The number of islets in the treatment groups (T1D-Autograft and T1D-Allograft) increased (6.3±1.5 islet and 6.0±1.0 islet, respectively) and there were no statistically significant differences between the two groups. This showed that both autograft and allograft cell transplantation could regenerate pancreatic islet numbers. However, the size of the pancreatic islets did not increase after transplantation. ( Figure 6A,B )
Figure 6.
Comparison of the size and number of pancreatic islets in diabetic mice following mADSC treatment
Although the size of the pancreatic islets did not increase, immunohistochemistry (IHC) staining results showed that pancreatic islets recovered their ability to secrete insulin. About 70% of cells in pancreatic islets are beta cells which are able to secrete insulin in response to blood glucose levels in mice. In islets of Normal-Placebo group, the positive staining of insulin was very clear, in contrast to the T1D-Placebo group. In the T1D-Autograft and T1D-Allograft groups, the injured pancreases were regenerated after ADSC transplantation. At day 56 post transplantation, insulin-positive cells appeared in the pancreases of treated mice. These results were confirmed by results of blood insulin levels in ELISA assays. The level of insulin that exist in the peripheral blood may reflect the regeneration of the pancreas after treatment. Insulin levels were found to be significantly different among the experimental groups ( Figure 7 , Figure 8 ). The results showed that the insulin concentration in treated mice (T1D-Autograft and T1D-Allograft) significantly increased compared to T1D-Placebo group (1.28±0.02 µg/L and 1.58±0.13 µg/L vs. 0.97±0.07 µg/L, respectively) ( Figure 8 ). These results showed that autograft and allograft ADSC transplantation helped to rehabilitate pancreas function in our diabetic mouse model.
Discussion
Treatment with ADSCs has been shown to have the potential to recover pancreas. In previous studies, ADSCs have served as an ideal candidate for T1D treatment worldwide Kono et al., 2014 Lin et al., 2009 Rahavi et al., 2015 Yaochite et al., 2015 . ADSCs have been used in clinical trials based on their ability to differentiate into beta cells and stimulate regeneration of beta cells in the damaged pancreas El-Badawy and El-Badri, 2016 . Fat tissue is an abundant source of MSCs. Among the different kinds of MSCs, the proliferation of stem cells derived from adipose tissue has been demonstrated to be better than cells from bone marrow tissue Semon et al., 2014 . Simultaneously, ADSCs has a greater potential for angiogenesis Dao et al., 2016 Vu et al., 2014 .
In this study, we have successfully cultured ADSCs according to a protocol from our laboratory Vu et al., 2015 . These cells exhibited the mesenchymal stem cell phenotypes. Indeed, they could spread over the surface of plastic flasks with fibroblast shape; successfully differentiated into adipocytes and osteoblasts. According to Dominici et al. (2006), the some markers for mesenchymal stem cells should be done, however we applied the previous protocol without modifications Vu et al., 2015 , these cells only were checked their shapes and differentiation potential.
In the next experiments, ADSCs were used to treat T1D mice in two platforms included autologous and allogenic transplantations. The results showed that ADSC transplantation successfully recovered number of islets, recovered pancreas functions of diabetic mellitus mouse, and reduced blood glucose levels. More importantly, there were not significantly different about efficacy of autologous and allogenic transplantation of ADSCs in diabetes mellitus treatment. This result showed that effects of ADSCs did not dependent on autologous ADSCs or allogenic ADSCs. Indeed, ADSCs as well as MSCs exhibited the low immunogenicity Gu et al., 2015 Yaochite et al., 2015 . Therefore, both autologous and allogenic ADSCs can exist in the models. Moreover, the effects of ADSCs can relate to some signals or growth factors that secreted by ADSCs. The secreted product of ADSCs involved in immune modulation, conditioned inflammatory process, protected the remaining beta cells and stimulated the proliferation of beta cells Fiorina et al., 2011 Kono et al., 2014 . Previous studies showed that the paracrine effects of ADSCs played important roles in controlling blood glucose in animal diabetic models. ADSCs could produce inflammatory cytokines and contribute to avoid stress due to high blood glucose Rahavi et al., 2015 .
Conclusion
In this study, we have successfully isolated and cultured mouse adipose derived stem cells which exhibit characteristics of MSCs, such as the ability to differentiate into osteoblasts and adipocytes. After transplantation of 2x106 mADSCs, mice showed recovery of pancreatic islets and improvement of insulin production of beta cells, thereby reducing blood glucose levels in mice. There was no difference between autologous (autograft) and allogeneic (allograft) transplantations of ADSCs. After treatment, blood glucose levels decreased and were stably maintained from day 35 to day 56, and mortality rate was decreased to 0%. Overall, our study shows that adipose tissue stem cells can reduce blood glucose levels and reactivate a functional part of the pancreas. Both autologous and allogeneic ADSCs are efficacious for treatment of T1D.
Abbreviation
MSCs: Mesenchymal stem cells
STZ: Streptozotocin
T1D: Type 1 diabetes mellitus disease
ADSCs: Adipose-derived stem cells
Author Contribution
Anh NTB, Oanh TKN, Ngoc KP isolated, confirmed and cultured ADSCs; Oanh TKN, Phuc VP analysed data, wrote and revised the manuscript; Anh NTB, Cong LTN and Dung TN produced the type 1 diabetes mellitus models by STZ; autograft and allograft transplantation of ADSCs; monitored blood glucose, glucose. Insulin tolerance assay and pancreas structure evaluation were carried out by BNT Anh; Loan TTD and Cong LTN.
References
- B.A. Bunnell, M. Flaat, C. Gagliardi, B. Patel, C. Ripoll. Adipose-derived stem cells: isolation, expansion and differentiation. Methods (San. 2008;Diego:Calif) 45, 115-120. Google Scholar
- T.T.-T. Dao, N.B. Vu, L.T. Phi, H.T.-N. Le, N.K. Phan, P. Van Pham. Human adipose-derived mesenchymal stem cell could participate in angiogenesis in a mouse model of acute hindlimb ischemia. Biomedical Research and Therapy. 2016;3:770-779. Google Scholar
- A. El-Badawy, N. El-Badri. Clinical Efficacy of Stem Cell Therapy for Diabetes Mellitus: A Meta-Analysis. PLoS ONE. 2016;11:e0151938. Google Scholar
- P. Fiorina, J. Voltarelli, N. Zavazava. Immunological applications of stem cells in type 1 diabetes. Endocrine reviews. 2011;32:725-754. Google Scholar
- L.H. Gu, T.T. Zhang, Y. Li, H.J. Yan, H. Qi, F.R. Li. Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell Mol Immunol. 2015;12:444-455. Google Scholar
- P.A. Halban, K.S. Polonsky, D.W. Bowden, M.A. Hawkins, C. Ling, K.J. Mather, A.C. Powers, C.J. Rhodes, L. Sussel, G.C. Weir. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes care. 2014;37:1751-1758. Google Scholar
- E. Karaoz, A. Okcu, Z.S. Unal, C. Subasi, O. Saglam, G. Duruksu. Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy. 2013;15:557-570. Google Scholar
- J. Katuchova, D. Harvanova, T. Spakova, R. Kalanin, D. Farkas, P. Durny, J. Rosocha, J. Radonak, D. Petrovic, D. Siniscalco. Mesenchymal stem cells in the treatment of type 1 diabetes mellitus. Endocrine pathology. 2015;26:95-103. Google Scholar
- T.M. Kono, E.K. Sims, D.R. Moss, W. Yamamoto, G. Ahn, J. Diamond, X. Tong, K.H. Day, P.R. Territo, H. Hanenberg. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: analysis of hASC-derived paracrine effectors. Stem Cells. 2014;32:1831-1842. Google Scholar
- D.S. Kwon, X. Gao, Y.B. Liu, D.S. Dulchavsky, A.L. Danyluk, M. Bansal, M. Chopp, K. McIntosh, A.S. Arbab, S.A. Dulchavsky. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. International wound journal. 2008;5:453-463. Google Scholar
- G. Lin, G. Wang, G. Liu, L.J. Yang, L.J. Chang, T.F. Lue, C.S. Lin. Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem cells and development. 2009;18:1399-1406. Google Scholar
- H.P. Lin, T.M. Chan, R.H. Fu, C.P. Chuu, S.C. Chiu, Y.H. Tseng, S.P. Liu, K.C. Lai, M.C. Shih, Z.S. Lin. Applicability of adipose-derived stem cells in type 1 diabetes mellitus. Cell transplantation. 2015;24:521-532. Google Scholar
- W.H. Organization. Global report on diabetes. World Health Organization. 2016;:. Google Scholar
- H.J. Paek, C. Kim, S.K. Williams. Adipose stem cell-based regenerative medicine for reversal of diabetic hyperglycemia. World Journal of Diabetes. 2014;5:235-243. Google Scholar
- H. Rahavi, S.M. Hashemi, M. Soleimani, J. Mohammadi, N. Tajik. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res. 2015;2015:878535. Google Scholar
- J.A. Semon, C. Maness, X. Zhang, S.A. Sharkey, M.M. Beuttler, F.S. Shah, A.C. Pandey, J.M. Gimble, S. Zhang, B.A. Scruggs. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem cell research & therapy. 2014;5:2. Google Scholar
- K. Timper, D. Seboek, M. Eberhardt, P. Linscheid, M. Christ-Crain, U. Keller, B. Muller, H. Zulewski. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochemical and biophysical research communications. 2006;341:1135-1140. Google Scholar
- N.B. Vu, A.N.-T. Bui, V. Ngoc-Le Trinh, L.T. Phi, N.K. Phan, P. Van Pham. A comparison of umbilical cord blood-derived endothelial progeni-tor and mononuclear cell transplantation for the treatment of acute hindlimb ischemia. . 2014;:. Google Scholar
- N.B. Vu, N.L. Van Trinh, L.T. Phi, T.L.H. Vo, T.T.T. Dao, N.K. Phan, T. Van Ta, P. Van Pham. An evaluation of the safety of adipose-derived stem cells. Biomedical Research and Therapy. 2015;2:22. Google Scholar
- J.N. Yaochite, C. Caliari-Oliveira, L.E. de Souza, L.S. Neto, P.V. Palma, D.T. Covas, K.C. Malmegrim, J.C. Voltarelli, E.A. Donadi. Therapeutic efficacy and biodistribution of allogeneic mesenchymal stem cells delivered by intrasplenic and intrapancreatic routes in streptozotocin-induced diabetic mice. Stem cell research & therapy. 2015;6:31. Google Scholar
- F. Zhou, Y. Hui, Y. Xu, H. Lei, B. Yang, R. Guan, Z. Gao, Z. Xin, J. Hou. Effects of adipose-derived stem cells plus insulin on erectile function in streptozotocin-induced diabetic rats. International Urology and Nephrology. 2016;48:657-669. Google Scholar
- P. Zuk. Adipose-Derived Stem Cells in Tissue Regeneration: A Review. ISRN Stem Cells. 2013;2013:35. Google Scholar
 Biomedpress
Biomedpress
 Open Access
Open Access 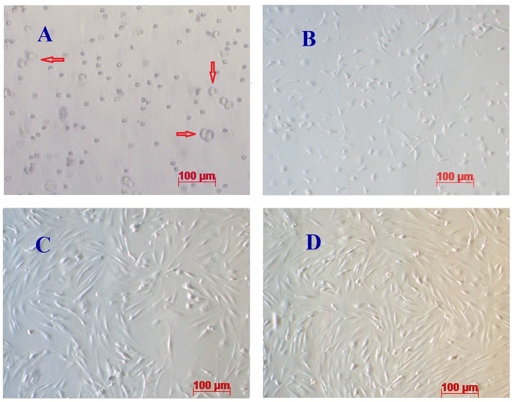
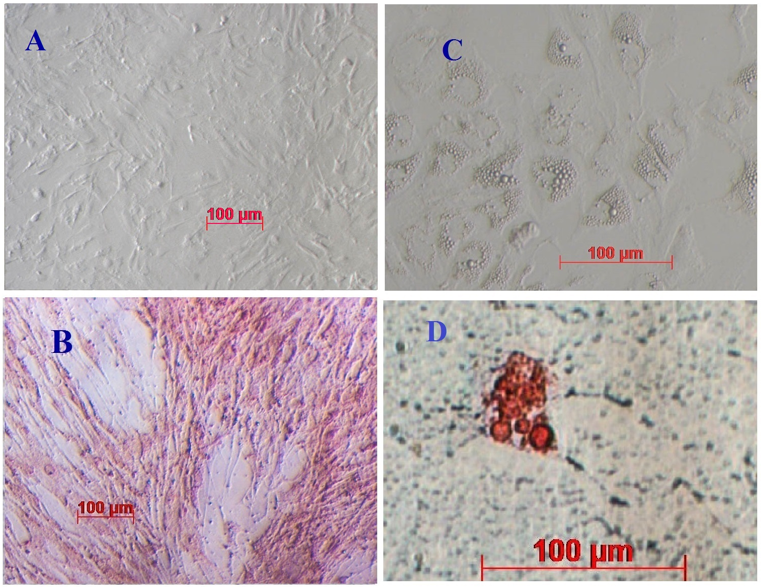
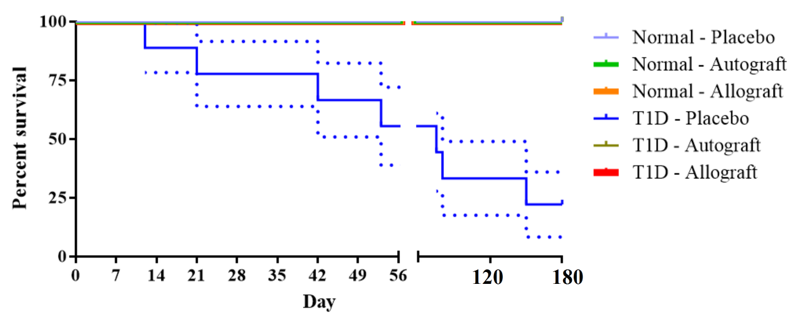
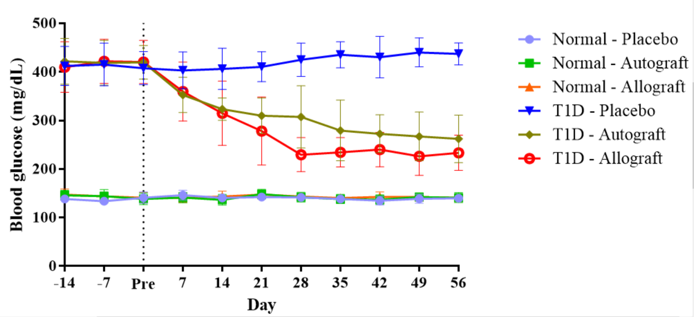
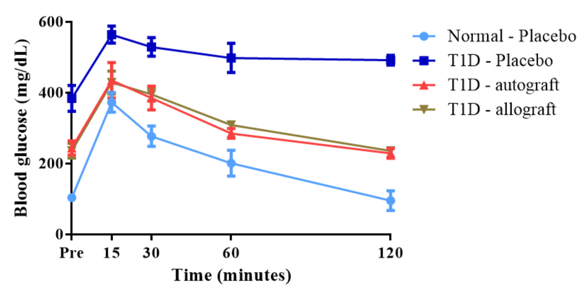
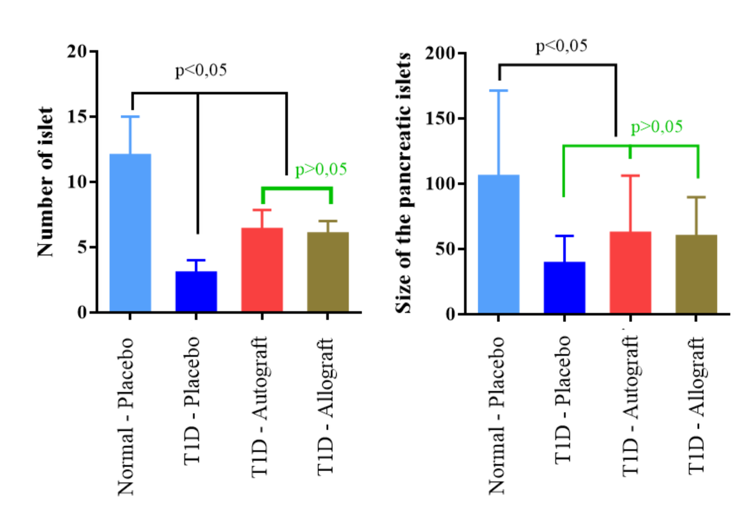
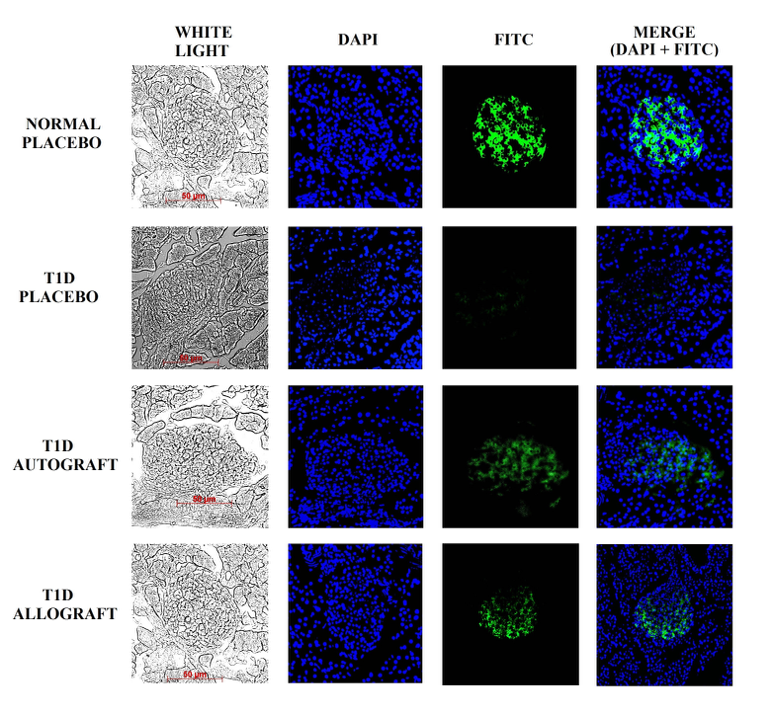
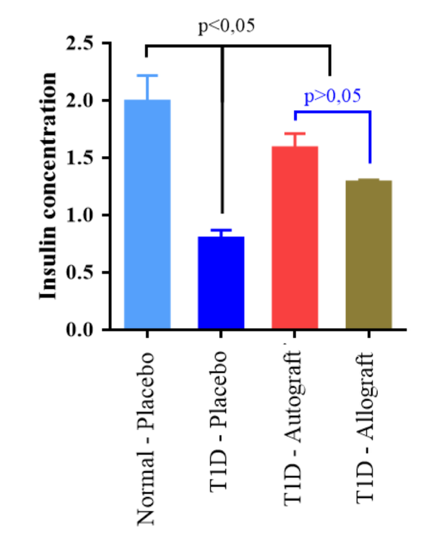







Figure 1