Abstract
Introduction: Both ETV-2 and HGF factors are known to be important factors which trigger neo-angiogenesis both in vitro and in vivo models. This study aimed to treat hindlimb ischemia in mice by co-injection of ETV-2 and HGF viral vectors.
Methods: ETV-2 and HGF vectors were prepared per previous publications. The mouse ischemic hindlimb model was performed by ligating and burning the artery at the hindlimb. ETV-2 and HGF viral vectors were co-injected into the ligated and burnt sites.
Results: The results showed that co-injection of the vectors significantly improved angiogenesis as well as reduced leg loss in mice compared to placebo treatment. The percentage of mice who succumbed to ischemia was also significantly reduced compared to control.
Conclusion: Overall, this study suggests a potential impact of combining ETV-2 and HGF to treat angiogenesis. Use of ETV-2 and HGF viral vectors can be a promising therapy for ischemia treatment in the future.
Introduction
Ischemia is a condition in which blood supply is restricted to tissues, causing a lack of oxygen and nutrients to cells. Ischemia can cause extreme complications, especially at the heart, brain and hindlimb. Today, there are several routine therapies used to treat this condition, including drugs and surgery. However, these therapies have met with limitations, especially when treating acute ischemia and in cases where numerous blood vessels are affected.
Based on previous promising results with gene therapy using ETV2 or HGF vector injections to treat ischemic hindlimb Kibbe et al., 2016 Van Pham et al., 2017 Van Pham et al., 2016b , we aimed to investigate if co-injection of ETV2 vector with HGF vector could improve blood neoangiogenesis and blood recovery in a mouse model of ischemic hindlimb.
Indeed, the E-twenty-six (ETS) factor variant 2 (ETV2) was determined to be an important transcription factor for ETS, controlling the formation of hematopoietic and vascular systems in both embryogenesis and postnatal development Lindgren et al., 2015 Liu et al., 2015 Sumanas and Choi, 2016 . Indeed, overexpression of ETV2 in some studies has successfully led to the reprogramming of certain kinds of cells into endothelial cells Ginsberg et al., 2012 Morita et al., 2015 Van Pham et al., 2016a .
Besides ETV2, hepatic growth factor (HGF) is also considered an angiogenic factor. A product (by the name of VM202) that contains the HGF vector has been tested in clinical trials for the treatment of critical limb ischemia. To date, clinical trials using this vector have demonstrated that injection of HGF vector, as opposed to placebo, can reduce ulcers Kibbe et al., 2016 . Moreover, in vitro studies have also demonstrated that HGF enhances the direct conversion of fibroblasts into EPC under ETV2-transducing conditions Van Pham et al., 2016b .
Materials-Methods
Mouse model of hindlimb ischemia
Twelve-month old mice were used for the ischemic hindlimb model. All animal protocols and experiments were prepared according to the “Guide for the Care and Use of Laboratory Animals” of the Laboratory of Stem Cell Research and Application, University of Science, VNU-HCM, Vietnam and approved by the Committee for Care and Use of Laboratory Animals. Acute hindlimb ischemic mice were established according to previously published protocols (Vu et al., 2014). Briefly, the mice were anesthetized using 7.5 mg/kg zoletil. Hairy thighs were shaved and then an incision (approximately 1 cm long) was made along the thigh skin. The fat thighs were removed and the femoral arteries near the abdomen were dissected from the veins and nerves, and ligated at two positions. Between the two ligated artery positions, a burn was made using an electronic cutting machine (ESU-X, Geister, Germany). Finally, the skin was stitched and the wound area was covered in povidone-iodine.
Production of ETV2 and HGF viral vectors
Production of the ETV2-expressing viral vector was performed as described in previous studies Van Pham et al., 2016a Van Pham et al., 2016b . Briefly, the human ETV2 & HGF expression vectors (pF1KB9707 & Plasmid #10901, respectively) were obtained from Addgene, Inc. (Cambridge, MA). ETV2 and HGF were cloned individually into the vector backbone pSIN4-EF1alpha-IRES-Puro (Plasmid #61061; Addgene) to generate pSIN4-EF1a-ETV2-IRES-Puro and pSIN4-EF1a-HGF-IRES-Puro, respectively. All of the coding sequences in the expression vector were confirmed using a GenomeLab System (Beckman Coulter, Brea, CA). The ETV2 and HGF vectors were then transfected into HEK293T cells along with pCMV-VSV-G-RSV-Rev and pCMV-dR8.2 (Addgene).
Eighteen hours after transfection, the medium was replaced with fresh culture medium. After 48 h of culture, the lentivirus-containing medium was collected, passed through a 0.45-μm filter and concentrated by centrifugation (8400 × g at 4°C for 16 h). The lentivirus pellets were resuspended in PBS at 107 IFU/mL for each.
Hindlimb ischemia treatment by viral vector injection in mice Mice with hindlimb ischemia were divided into 2 groups (n=12 per group). In Group I (G1; placebo group) mice were injected with empty viral vector. In Group II (G2; treatment group), mice were injected with a dose of 107 IFU/mL ETV2 virus in 100 µL and 107 IFU/mL HGF virus in 100 µL. The viral vectors were directly injected into the muscle at the burn sites at day 0. Following injection, all mice were monitored up to 30 days to evaluate the effect of the grafted cells.
Necrosis grade of hindlimb ischemia
The degree of ischemic damage was assessed by the grade of limb necrosis according to the guidelines of Goto et al. Goto et al., 2006 . Ischemia was scored according to the following grades: Grade 0- normal limb without swelling, necrosis or atrophy of muscle; Grade I- necrosis limited to the toes; Grade II- necrosis extending to the foot; Grade III- necrosis extending to the knee; and Grade IV- necrosis extending to the hip or loss of the whole hindlimb.
SpO2 measurement
Oxygen saturation (SpO2) measurement was used to evaluate blood recovery. In this study, SpO2 measurements in the toes were recorded at various timepoints: before burning the blood vessels, on the day of treatment (day 0) with viral vector, and 3 d or 30 d after treatment. SpO2 was measured with a pulse oximeter (Omron, Osaka, Japan).
Trypan blue assay
The trypan blue flow assay was used to evaluate blood flow. Briefly, 1% trypan blue was injected into the tail vein. If blood vessel recovery occurred at the burn sites, the trypan blue dye would be delivered to the toes and feet, causing them to stain blue. The time needed for staining of toes and feet after injection of trypan blue was measured with a stopwatch (in seconds).
Statistical analysis
Statistical analysis of all endpoints was performed using the two-sided Student’s t test or one-way analysis of variance. All data were presented as mean ± SD; p < 0.05 was considered statistically significant. Data were analyzed using Prism 6.0 software (GraphPhad Prism, La Jolla, CA).
Results
Production of ischemia murine model
Blood flow after injury
After 20s of trypan blue injection, all mice with normal hindlimbs stained blue with trypan blue, indicating a normal blood flow. The strongest staining was recorded 1 h after injection. However, for the group of mice with ligated blood vessel in the hindlimb, it was observed that the hindlimb (particularly feet) did not stain blue after dye injection ( Table 1 , Figure 1 ). After 6 d, all mice with intact legs (auto-recovery) and normal legs could be stained with trypan blue. However, in the injured legs, the stain was lighter than in the normal legs.
Table 1.
Figure 1. Blood flow to hindlimb before and after injury
Trypan blue injection did not stain the injured hindlimb (blue arrows), but stained the normal hindlimb (yellow arrow) at 1 day (A), 6 days (B) and 28 days (C) after dye injection.
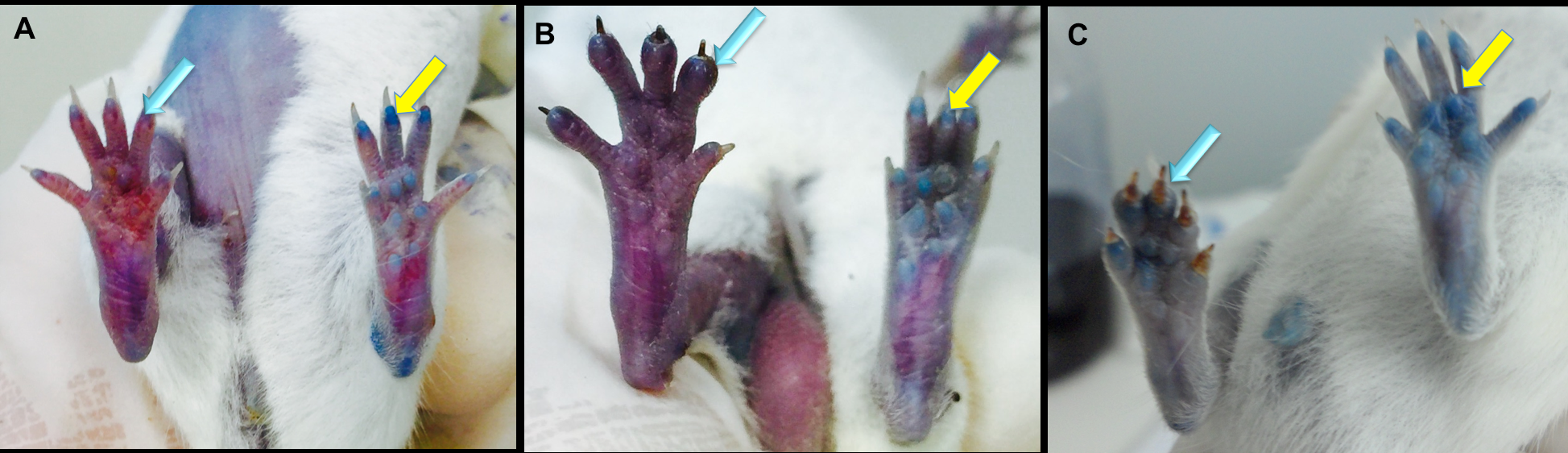
Edema after injury
After 3 d of ligation of the artery, almost all mice had edema and redness. The edema level was evaluated by the ratio of wet tissue to dry tissue. Edema in ligated mice was significantly higher than in normal mice (4.59 ± 0.18 in ligated mice vs 3.45 ± 0.09 in normal mice, respectively) (p<0.05). These results indicate that artery ligation can cause hindlimb ischemia. After 7 d, all ligated mice were able to recover automatically without leg less and the level of edema in ligated mice and normal mice was not significantly different ( Figure 2 ).
Figure 2. Assessment of edema in the ischemic hindlimb
A. Edema appeared in the artery of ligated hindlimb. B. The level of edema was evaluated for mice in ligated mice and normal mice (p<0.05).
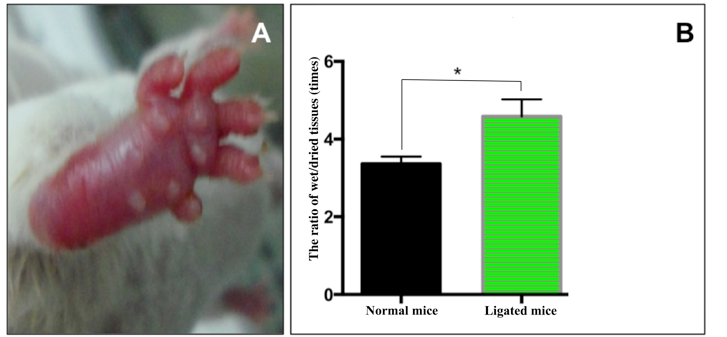
Histology analysis
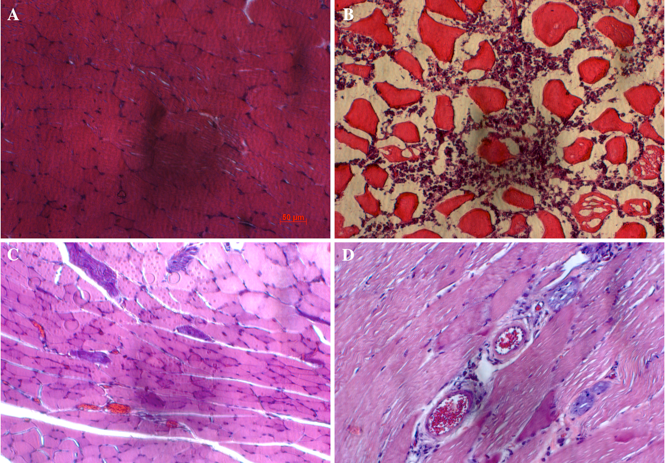
The histological results are presented in Figure 3 . For normal mice, the muscle cells were arranged in an orderly fashion with cellular nuclei at the perizone of cytoplasm. For muscles obtained from artery-ligated hindlimb of G1 and G2 mice, the tissue structure changed significantly after 3 d ( Figure 3 ). Indeed, the cellular nuclei in these tissues concentrated into clusters and the cytosol of the cells were damaged. By naked eye observation, new blood vessels were formed in the ligated mice. Notably, angiogenesis could be clearly observed around 28 d after ligation.
Figure 3. Histological evaluation of muscles
Shown are muscles of normal mice (A) and of ligated mice at 3 d (B) and 28 d (C) after treatment. Yellow arrow: cell nuclei; white arrow: cytoplasm; blue arrow: blood vessels; green arrow: inflammation niches; and red arrow: adipocytes.
Necrotic grades
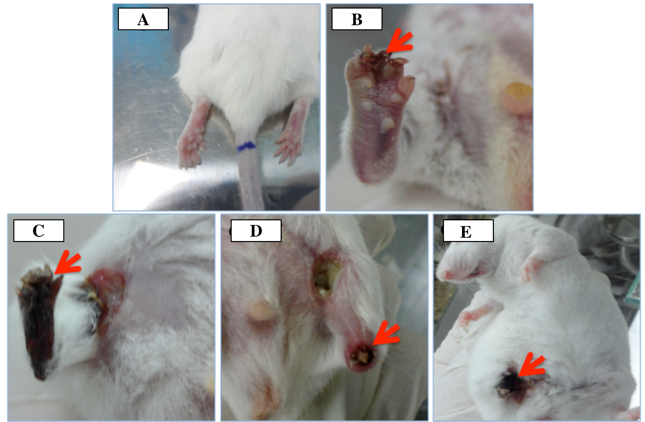
The necrotic grades of hindlimbs were evaluated based on the guidelines of Takako Goto et al. Goto et al., 2006 . After 3 h of ligation, mice exhibited features of injury including decrease of hindlimb movement. The injury became more serious after 3 h of monitoring. At day 7, we evaluated the necrotic grade of the hindlimbs for the ligated mice and normal mice. In the ligated group, up to 100% (30/30) of the mice exhibited necrosis at the hindlimb; of these, 90% showed grade III & IV necrosis (50% grade III and 40% mice grade IV) while only 10% mice showed grade I ( Figure 4 ). These observations did not change even out to day 28 of monitoring.
Figure 4. Necrosis of hindlimb after artery ligation
(A) Normal, (B) Grade I, (C) Grade II, (D) Grade III, (E) Grade IV)
Co-injection of ETV-2 and HGF reduced hindlimb necrosis
The effect of co-injection of ETV2 and HGF lentiviral vectors on the recovery of ischemic hindlimb in mice was observed for 30 d. The grade of limb necrosis was evaluated and classified according to the guidelines of Goto et al. The results suggest that injection of ETV2+HGF lentiviral vectors can help rescue or treat hindlimb ischemia. Indeed, in the GII group (ETV2+HGF injection), 66.67% (8/12) of mice completely recovered (grade 0), 8.33% (1/12) had necrotic grade I, 0% had necrotic grade II, III or IV, and 25% (3/12) died. In the GI group (empty viral vector, placebo injection), only 8.33% (1/12) of mice completely recovered, 41.67% (5/12) had necrotic grade I, 0% (0/12) had necrotic grade II and III, 8.33% (1/12) had necrotic grade IV, and 41.67% (5/12) died. These results demonstrate that injection of both ETV2 and HGF viral vectors can induce full recovery of mice toward grade 0 by 8-fold (66.67% vs. 8.33%, for GII vs GI, respectively). The death rate of mice also significantly decreased (41.67% vs. 25%; for GI vs. GII, respectively).
Co-injection of ETV-2 and HGF increased blood flow
The SpO2 and trypan blue assays support the observations that blood flow of treated mice was increased. The SpO2 results showed that after injection with ETV2 viral vector, SpO2 in the GII group increased more than the GI group; however, there was no statistical difference in SpO2 at day 3 or day 30. On the contrary, there was an observed significant difference in SpO2 between normal mice and GI/GII mice.
The trypan blue assay showed that in surviving mice without leg loss, the average time for staining of toes and feet with trypan blue was significantly different between GI and GII, and compared to normal mice. In normal mice and in those with normal hindlimb, the feet and toes of the mice turned blue 20 s after trypan blue injection into the tail vein. In the treated group, ischemic hindlimbs required 143±37.45 s to turn blue at day 10, 30 s to turn blue at day 10, and 25.25 s to turn blue at day 30. In the control group, ischemic hindlimbs required 25.77 ± 5.66 s, 20s and 20 s to turn blue at day 10, 20 and 30, respectively.
Change in histology of hindlimb muscles
Tissue necrosis occurred in Group I mice and group II mice at day 3 after ligation and treatment. Indeed, the muscle sections showed cellular cytoplasm shrinking. Moreover, cells were not arranged in a particular order, with nuclei randomly aligned along the edge of cells (plasma membrane) and concentrating near or in the cytoplasm. However, after 30 d of treatment, the tissue structure of Group I mice and group II mice remained intact; mice did not lose their legs and survival was significantly improved ( Figure 5 ).
Figure 5. The changes in histology of hindlimb muscles after treatment
The normal muscle tissue (A) changed the histological structure after ligation and treatment at day 3 (B), day 10 (C) and day 30 (D).
Discussion
To our understanding, this is the first study to evaluate co-injection of ETV2 and HGF to treat ischemic hindlimb. The study results showed that co-injection of the 2 vectors (ETV2 and HGF) significantly improved angiogenesis in the mouse ischemic hindlimb model and could improve blood vessel recovery.
Our results showed that following induction of ischemia hindlimb in mice, only 8.33% (1/12) mice could auto-recover; their necrotic grade moved towards grade 0 after 28 d. Meanwhile in mice treated with ETV2+HGF lentiviral vectors, 66.67% (8/12) mice showed recovery towards grade 0 after 28 d of treatment. Moreover, the death rate of mice was also significantly reduced- from 41.67% in the placebo group (G1) to 25% in the treated group (G2). These data demonstrate the impact of EVT2 and HGF in neo-angiogenesis at ischemic hindlimbs.
The results of the SpO2 and trypan blue assays further corroborate the occurrence of new blood vessel formation to provide oxygen and blood to downstream tissues. The SpO2 values gradually increased as the time of trypan blue staining of toes decreased, thereby also supporting the above conclusion. Blood vessel recovery also helped muscle tissue regeneration and healing. Furthermore, H&E staining showed that muscle tissues at the ligated and burnt sites were capable of regenerating and healing.
Overall, our results confirm the important role of ETV2 and HGF. In a previous study Van Pham et al., 2017 , we showed that ETV2 alone could aid in treating ischemic hindlimbs by activation of endothelial cell proliferation, leading to attenuated acute hindlimb ischemia in mice Van Pham et al., 2017 . In this study, our preliminary data show that ETV2 expression impacts angiogenesis of endothelial cells. When ETV2 was over-expressed, it could stimulate proliferation of local skeletal endothelial cells. Our observations reconfirmed similar observations by Park et al. (2016) Park et al., 2016 . In their study, Park and colleagues also showed that a vector carrying ETV2, after injection into ischemic hindlimbs, could improve the recovery of blood perfusion with enhanced vessel formation Park et al., 2016 . The role of ETV2 in endothelial differentiation and development has also been documented in other studies Lindgren et al., 2015 Liu et al., 2015 Sumanas and Choi, 2016 Van Pham et al., 2016a .
The combination of HGF and ETV2 in the treatment of ischemic hindlimb is advantageous and possibly synergistic. There have been previous observations that HGF can strongly increase the efficacy of direct reprograming of fibroblasts into endothelial progenitor cells via ETV2 transduction Van Pham et al., 2016b .
Indeed, in that study, the direct reprogramming efficacy by ETV2 transduction increased from 5.41±1.51% for ETV2 transduction alone to 12.31±2.15% for ETV2 transduction combined with HGF treatment Van Pham et al., 2016b . It is perhaps their synergistic action that a full recovery of ischemic hindlimbs was observed in this study; compared to previous studies in which 58.33 % of mice showed full recovery (grade 0) with ETV2 treatment alone Van Pham et al., 2017 , in our study 66.67% of mice showed full recovery (grade 0) with ETV2 and HGF co-treatment. Indeed, under angiogenic conditions, combining VEGF-A with HGF can promote neovascularization Sulpice et al., 2009 Xin et al., 2001 . HGF also can induce capillary morphogenesis of endothelial cells through Src Kanda et al., 2006 .
Conclusion
Ischemia is an important health condition that can cause injury for many organs, including brain, heart and hindlimb, due to disruption of blood flow to those downstream tissues/organs. In this study, we show that injection of both ETV2 and HGF lentiviral vectors could significantly stimulate angiogenesis, leading to reduced mortality of mice with ischemic hindlimb. Blood vessel recovery after vector injection improved hindlimb loss, increased blood flow to the toes, and regenerated muscle tissues. Overall, the results of this study suggest that gene therapy with ETV2 and HGF is a promising platform for the treatment of ischemia, particularly hindlimb ischemia.
Author Contribution
PVP performed the cell culture, vector preparation; analysis the results and wrote the manuscript. NBV, TTTD, NTNL, LTP, NKP carried out the ischemia models, treatment with HGF, ETV2 vectors, harvested the data.
References
- M. Ginsberg, D. James, B.S. Ding, D. Nolan, F. Geng, J.M. Butler, W. Schachterle, V.R. Pulijaal, S. Mathew, S.T. Chasen. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151:559-575. Google Scholar
- T. Goto, N. Fukuyama, A. Aki, K. Kanabuchi, K. Kimura, H. Taira, E. Tanaka, N. Wakana, H. Mori, H. Inoue. Search for appropriate experimental methods to create stable hind-limb ischemia in mouse. Tokai J Exp Clin Med. 2006;31:128-132. Google Scholar
- S. Kanda, H. Kanetake, Y. Miyata. HGF-induced capillary morphogenesis of endothelial cells is regulated by Src. Biochem Biophys Res Commun. 2006;344:617-622. Google Scholar
- M.R. Kibbe, A.T. Hirsch, F.O. Mendelsohn, M.G. Davies, H. Pham, J. Saucedo, W. Marston, W.B. Pyun, S.K. Min, B.G. Peterson. Safety and efficacy of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with critical limb ischemia. Gene Ther. 2016;23:399. Google Scholar
- A.G. Lindgren, M.B. Veldman, S. Lin. ETV2 expression increases the efficiency of primitive endothelial cell derivation from human embryonic stem cells. Cell Regeneration. 2015;4:1. Google Scholar
- F. Liu, D. Li, Y.Y.L. Yu, I. Kang, M.J. Cha, J.Y. Kim, C. Park, D.K. Watson, T. Wang, K. Choi. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO reports. 2015;16:654-669. Google Scholar
- R. Morita, M. Suzuki, H. Kasahara, N. Shimizu, T. Shichita, T. Sekiya, A. Kimura, K. Sasaki, H. Yasukawa, A. Yoshimura. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci U S A. 2015;112:160-165. Google Scholar
- C. Park, T.J. Lee, S.H. Bhang, F. Liu, R. Nakamura, S.S. Oladipupo, I. Pitha-Rowe, B. Capoccia, H.S. Choi, T.M. Kim. Injury-Mediated Vascular Regeneration Requires Endothelial ER71/ETV2. Arterioscler Thromb Vasc Biol. 2016;36:86-96. Google Scholar
- E. Sulpice, S. Ding, B. Muscatelli-Groux, M. Berge, Z.C. Han, J. Plouet, G. Tobelem, T. Merkulova-Rainon. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell. 2009;101:525-539. Google Scholar
- S. Sumanas, K. Choi. ETS Transcription Factor ETV2/ER71/Etsrp in Hematopoietic and Vascular Development. Curr Top Dev Biol. 2016;118:77-111. Google Scholar
- P. Van Pham, N.B. Vu, H.T. Nguyen, T.T.-T. Dao, H.T.-N. Le, L.T. Phi, O.T.-K. Nguyen, N.K. Phan. ETV-2 activated proliferation of endothelial cells and attenuated acute hindlimb ischemia in mice. In Vitro Cellular & Developmental Biology - Animal. 2017;:. Google Scholar
- P. Van Pham, N.B. Vu, H.T. Nguyen, O.T. Huynh, M.T.-H. Truong. Significant improvement of direct reprogramming efficacy of fibroblasts into progenitor endothelial cells by ETV2 and hypoxia. Stem Cell Research & Therapy. 2016a;7:104. Google Scholar
- P. Van Pham, N.B. Vu, M.T.-H. Truong, O.T. Huynh, H.T. Nguyen, H.L. Pham, N.K. Phan. Hepatocyte growth factor improves direct reprogramming of fibroblasts towards endothelial progenitor cells via ETV2 transduction. Biomedical Research and Therapy. 2016b;3:45. Google Scholar
- X. Xin, S. Yang, G. Ingle, C. Zlot, L. Rangell, J. Kowalski, R. Schwall, N. Ferrara, M.E. Gerritsen. Hepatocyte Growth Factor Enhances Vascular Endothelial Growth Factor-Induced Angiogenesis in Vitro and in Vivo. The American Journal of Pathology. 2001;158:1111-1120. Google Scholar
 Biomedpress
Biomedpress
 Open Access
Open Access 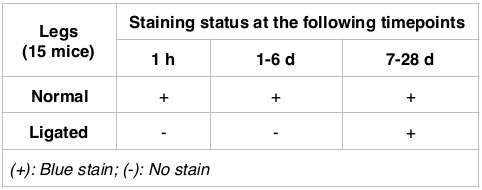







The staining of trypan blue in ligated legs