Abstract
Stem cells are vital for regenerating and repairing the organs such as the heart, lungs, skin, germ cells and other tissues. But the characteristics of normal stem cells and the discovery of origin of leukemia have headed the scientist to the hypothesis that cancer may initiate from stem cell-like cells. Cancer stem cells are now thought to be responsible for cancer initiation, progression, metastasis, recurrence and drug resistance. There are some similarities and differences between CSCs and healthy stem cells in signaling pathways (Wnt/ β–catenin, Hh, Notch), epigenetic modifications, telomere maintenance, and degree of dependence on the stem cell niche, maintenance of multipotency and self-renewability of respective progenitor cells. Distinct and specific surface biomarker phenotypes can be used to distinguish CSC from other tumor cells and normal stem cells through single and combination of markers such as CD44, CD34+CD38− and CD133. Recently, miRNAs have been tried as a marker and therapeutic agents against cancers stem cell such as miR-34a, miR-199a, miR-181, miR-125b-2 and miR-128. However, targeting CSCs without harming their normal counterparts is an advantageous way in the design of novel therapies but many questions remain unknown.
Introduction
Stem cell research is one of the most fascinating areas of contemporary biology because SCs have the potency to regenerate and repair the organs such as the heart, lungs, skin, germ cells and other tissues. In the 3- to 5-day-old blastocyst, the inner cells differentiated and developed to the entire body and organs Hongbao and Ma, 2014 . Given their exclusive regenerative aptitudes, SCs offer new potentials for treating diseases such as diabetes, primary immune deficiencies, aplastic anemia, severe haemoglobinopathies, cartilage and bone disorders, neurological diseases, skin and skin-appendage disorders and heart disease Strulovici et al., 2007 . By definition, SCs are necessarily undifferentiated cells of a multicellular organism that have the ability to proliferate themselves, going through the self-renewal and to produce mature cells of a particular tissue type (same and other) under highly controlled microenvironment. So far, SC self-renewability is maintained by two mechanisms namely obligatory asymmetric replication and stochastic differentiation Morrison and Kimble, 2006 . However, the features of SCs and the discovery of the link of leukemia (AML) initiation with CSCs have changed the way of thinking about the origin of cancer that cancer may initiate and contain self-renewal stem cell-like cells Han et al., 2013 Logtenberg and Boonstra, 2013 . The CSCs of leukemic blasts in AML initiated from antecedent cells in the bone marrow Dickson et al., 2014 . Surprisingly, AML mutated cells are found to be highly resistant to chemotherapy and are responsible for the recurrence of AML after an initial complete retardation, even after chemotherapy they rapidly replenish the leukemic cell population Yu et al., 2012 .So, AML- CSCs are those specific types of cells which initiate, progress cancer and somehow sustain in cell populations. Phenotypically, the cells (SC and CSC) in subpopulation look the same under the microscope but express a unique combination of surface molecules Logtenberg and Boonstra, 2013 Tang, 2012 . Cancer stem cells have self-renewal properties but with defectively regulated niche that lead to differentiate and create tumor, can metastasize eventually to remote site. Moreover, CSCs are able to avoid immune system and resistant against chemotherapy Sengupta and Cancelas, 2010 Zhou et al., 2009 Zhou et al., 2014 . Understanding the role of different pathways, epigenetic regulations and niche condition for initiation of progenitor cancer stem cell and identification of CSCs within cancer cell population is essential for the development of anticancer therapy, because diverse populations of cancer cells inside a tumor can switch back and forth between one cell that divide quickly but do not attack, and stem cell-like cells split deliberately but are extremely metastatic Kantara et al., 2014 . The intention of this review is to focus on the differences and similarities between stem cells andcancer stem cells, role of stem cell niches and epigenetic regulation and finally we discuss the current research on CSCs molecular markers and strategies for CSCs therapy, in brief.
Cancer Stem Cells and Stem Cells
Cancer stem cells have been defined as a small subpopulation of cancer cells within cancer microenvironment and niche that establish a reservoir of self-sustaining cells with the exclusive ability to self-renew and to form the heterogeneous lineages of cancer cells that comprise the tumor Logtenberg and Boonstra, 2013 . It could also be defined as likely all SSCs, CSCs are unspecialized (they have no unique tissue-specific structures), they can divide and renew themselves for long periods and they can give rise to specialized cells. They can therefore recapitulate tumor heterogeneity as they can be found in tumors. However, increasing evidence has showed that there are more similarities between CSCs and normal SCs Reya et al., 2001 Shackleton, 2010 . One of the prominent similarity between healthy stem cells and CSCs behavior is of EMT that via an alteration from a polarized epithelial cell type to a non-polarized mesenchymal cell type, CSCs are derived with SCs properties including migratory, self-renewal and differentiation properties Eridani, 2014 Martin-Belmonte and Perez-Moreno, 2011 . One of the differences between normal stem cells and cancer stem cells is their degree of dependence on the stem cell niche, a specialized microenvironment in which stem cells reside. Cancer stem cells may involve deregulation or alteration of the niche by dominant proliferation- promoting signals whereas normal SC has support and been supported by niche which provides homeostasis maintenance Li, 2006 . Chromosomally normal SCs are stable containing a normal diploid genome but CSCs are aneuploid with chromosomal rearrangements. In addition, normal stem cells are generally quiescent or very slow growing, reside in a specific niche, and have relatively long telomeres. Contrariwise, cancer cells expressing stem cell markers are not completely quiescent, and importantly, cancer stem/initiating cells have short telomeres which may reflect the multistep nature of cancer initiation and progression Shay and Wright, 2010 . Based on the frequency of occurrence, healthy stem cells are present in the tissues in a very small amount but CSCs can be a rather large population in the bulk of tumor cells Jaworska et al., 2015 . In addition to their ability to self-renew and differentiation, CSCs are also enriched in cells postulated to be resistant to conventional radiation and chemotherapy but not SCs. There are pieces of evidence to suggest that progenitor in both normal and cancer tissues are maintaining similar types a hierarchy. Cancer stem cells are mostly quiescent, since they replicate very slowly, while the progenitor cells grow and replicate much faster. The difference can easily demonstrate in vitro, especially in the terms of serum. Cancer stem cell culture in plastic plate does not allow cell adherence in the presence of growth factors (EGF and/or LIF) with serum free medium. The differentiated cells die within a few passages under such conditions. Contrary, in presence of serum, only a fraction of CSC can survive in culture dish. Interestingly, in serum free media, sometimes CSC can differentiate into progenitor cells House et al., 2015 . Table 1 represents the similarities and differences between cancer stem cells and stem cells.
Table 1.
Theories of the Origin of Cancer Stem Cell
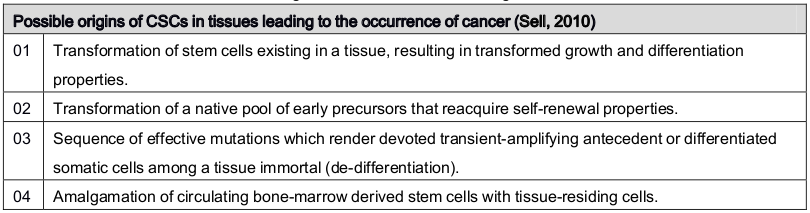
A single cancer cell has the ability to give rise to fresh cancer and engender heterogeneous progeny which provides proof for a stem-cell origin of cancer Ghaffari, 2011 . In the first probability, if a normal SC develops into CSC that indicates that it has already got a full stem-cell package notably differentiated into several progenitor cells, defense from immune surveillance, and emigrant in fur-flung sites. Initiation of cancer from stem cell is started when a SC has “gone bad” and therefore the essential stem-cell properties of a cancer cell are commonly inherited Nguyen et al., 2012 . The second possibility, CSCs developing from any cell which gains the characteristics of stem cell is fortified with a partial stem-cell package. And eventually, it gets malignant behavior Pattabiraman and Weinberg, 2014 . So, cancer forms as a result of acquiring the wrong ways of a stem cell by a good cell. Theoretically, when a differentiated cell gains vital components of SC it becomes dedifferentiated and cancerous Chambers and Werb, 2015 . Last but not least, the third option, when cancer encompasses simply deportee normal SCs it gets a separate package. And the association of bad cell with a good SC turns into cancer. In this phenomenon, CSCs are attracted by normal stem cells thus which ultimately plays the vital role in establishing and improvement of the malignancy. A very common interrogation about the theory of a stem-cell origin of cancers is whether cancer results from stem cells or it is determined by cells that have stem cell–like properties Takahashi et al., 2007 . Table 2 summaries the possible theories of the origin of cancer stem cell.
Table 2.
The theories of origins of CSCs in tissues leading to the occurrence of cancer
Tumor stem cell theory supported the Goldie-Coldman hypothesis and has the ability to explain how patients with metastatic disease display clinical deterioration after several months of starting treatment Martin, 2013 . That is due to the persistence of a small group of cells with unique characteristics, including the capacity to give rise to a new population of cells with resistant phenotype. This theory proposes that among all cancerous cells,very few show the functions of stem cells and replicate themselves to continue the cancer, very similar to healthy stem cells that usually renew and sustain our organs and tissues Yoo and Hatfield, 2008 . In this view, cancer cells that are not stem cells cannot sustain against drug over the long term. However, in order for a cell to become cancerous, it must undergo a significant number of critical alterations in the DNA sequences that control the cell Mayer et al., 2015 .
Cancer Stem Cells Niche
Cancer stem cells frequently seem to exist in and influenced by its microenvironment and niche which comprised with different molecular signals provided by neighboring connective tissue cells such as mesenchymal, vascular cells, adult SCs, and stromal cells which control their self-renewal and differentiation. A specific example is that CSCs was found at the interface of tumor and stroma in squamous cell carcinoma, colorectal and bladder cancer Atsumi et al., 2008 He et al., 2009 Vermeulen et al., 2010 . In Brain CSCs the niche regulated the angiogenesis Li, 2006 . Leukemic CSCs mice cell found to be competed with HSCs for their normal osteoblastic and vascular niches in culture Pattabiraman and Weinberg, 2014 . As a result, normal HSCs experience lack of support and reducing in number Colmone et al., 2008 . Tumor-associated monocytes and macrophages reported as a CSC niche component which functioned through juxtacrine signaling. Interaction of CSCs and CAFs in their niche found to maintain basal cell or squamous cell carcinoma in culture. In some malignant melanoma, keratinocytes also contribute as microenvironmental factor Lacina et al., 2015 . Many studies reported that Glioma stem cells (GSCs) reside in particular niches to maintain their behavior Chen et al., 2015 Lathia et al., 2015 . A hypoxic microenvironment has been reported to be important in regulating GSC molecular and phenotypic features through the recruitment of vascular and stromal cells Persano et al., 2013 . Thus, the microenvironment seems to be crucial for maintaining the properties of CSCs, preserve their phenotypic plasticity, protect them from the immune system, and facilitate primary tumor growth and their metastatic potential.
Wnt/β –catenin, Hh, Bmi-1, NF-κB, MAPK, Notch Signaling Pathway: Cancer Stem Cell
The signaling pathways those regulate self-renewal and differentiation of CSCs are not well understood. However, it seems that there are some overlap in the key signaling pathways between CSC and normal adult SCs Visvader and Lindeman, 2012 . Here we summarized some of these signaling pathways such as Wnt/β-catenin, Hedgehog (Hh), Bim 1 and Notch signaling those play a vital role in regulating CSCs. A good number data supported the functional versatility of Wnt/β-catenin signaling in SCs of the embryo and of the adult as well as in the CSCs Reya et al., 2001 . Wnt signaling found to be functioned as a niche factor to maintain SCs in a self-renewing state. Additionally, isolated Wnt proteins are active in a variety of stem cells, including neural, HSC, mammary and ESC Nusse, 2008 . Moreover, Wnt signaling and β-catenin have been shown to cause strong effects on ESCs both in terms of stimulating the expansion of stem cells and provoking differentiation into lineage determined cell types Merrill, 2012 . Nevertheless, it is well known that deregulation or abnormal activation of Wnt/β -catenin pathway may lead to a wide range of tumorigenesis, again, some proteins of Wnt signal transduction pathway are found to have tumor suppressive activity Luu et al., 2004 . In particular, Axin and APC mutations have also been recognized in some tumors, featuring the deregulation of this pathway in human cancer. The Hedgehog signaling pathway plays an essential role in patterning of development and maintenance of proliferative niches of stem cell and carcinogenesis Beachy et al., 2004 . Particularly, deregulated expression of components of theHh signaling pathway have been observed in a variety of range of hematological malignancies and decide CSC behavior directly or indirectly via the stromal microenvironment Copland and Campbell, 2015 .There are two models on maintenance of tumor growth or initiation by Hh ligands. The first one anticipation is that cancer cells generate Hh ligands and/or their stem cell renewal within the tumor which is sustained by the stromal environment Jiang and Hui, 2008 . Another postulation is that first Hh pathway in the stromal microenvironment activated then tumor growth is prompted Yauch et al., 2008 . Recently it has been identified that Bmi-1 is a vital regulator of the self-renewal of both ordinary, leukemic SCs and neuronal SC. It has also been suggested that there is a link between Bmi-1 and mammary carcinogenesis. Although Bmi-1 mediated stem cell self-renewal mechanism was not clear but P-16 silenced by Bmi-1 DeSano and Xu, 2009 . But, P-16 partially mediates the effects of Bmi-1 proteins in neural stem cells, which suggests that other factors might take part in Bmi-1’s effects on SC self-renewal Liu et al., 2005 . Notch-mediated cell-to-cell signaling has participation in various wings of embryonic development and regulation of tissue homeostasis in a variety of adult tissues, and controls stem cell maintenance, cell differentiation and cellular homeostasis Sancho et al., 2015 . Although Notch was originally identified as an oncogene, studies have also demonstrated that components of Notch may have growth-suppressive functions in some hematopoietic cells. A recent study demonstrated that CSC can be derived from human mammary epithelial cells followed by the activation of the Ras-MAPK pathway. The acquisition of these stem and tumorigenic characters is driven by EMT induction. MAPK/ERK1, 2 and vascular endothelial growth factor 1 (VEGF/Flt1) autocrine pathways may play substantial roles in drug-induced increase of bone marrow side-population cells. siRNA inhibition of Flt1 reduced nanog and Oct-4 expression, suggesting that stress-induced activation of the VEGF/Flt1 and MAPK/ERK1,2 autocrine loop may play an important role in the expansion of the CSC fraction Dayem et al., 2010 .It is well known that NF-κB pathway regulates cellular processes such as proliferation, stress response, innate immunity, and inflammation. NF-κB signaling is also important for the maintenance of pluripotency in human ESCs. The hypothesis “Transformation of SCs into CSCs as a result of extended oxidative stress, likely because of molecular modifications for example hypoxia-induced NF-κB” is supported by all of these findings Kennedy et al., 2007 . Although, different signaling pathways those control SC self-renewal and other properties have been described separately. But it is clear that in vivo there are wide interactions and crosstalk, between the pathways which are well recognized both in CSCs and stem cells, is still to find out and connect.
Epigenetic Regulation in Cancer Stem Cell
It is well established that cancer pathogenesis is strongly associated with abnormal epigenetic alterations such as losses and gains of DNA methylation, aberrant patterns of histone modifications and chromatin remodeling Plass et al., 2013 . These epigenomic modifications allow for the stable inheritance of various deviant cellular stuff without involving changes in DNA sequence or the amount of DNA in cancer cells. Recent studies on cell fate specification of embryonic and adult stem cells/progenitors have emphasized a general and critical role for dynamic epigenetic regulation in stem cell self-renewal and differentiation Wu and Sun, 2006 .Pluripotency of stem cells maintains high orders of epigenetic reprogramming events such as de novo methyltransferases and histone methyltransferases, making PSC as excellent candidates for analysis of epigenetic reprogramming during in vitro lineage-specific differentiation. This is possible that while DNA methylation might be critically involved in stopping alternative lineage differentiation genes in tissue-specific stem cells and/or differentiated cells, histone modification-mediated gene inactivation are principally involved to maintaining the self-renewal property and silencing of lineage differentiation genes inPSCs via Yamanaka factor (magic four) complex Patra et al., 2010 . In neural stem cell REST interact with the CoREST/LSD1 complex, that can repress neuronal gene promoters in the stem/progenitor cells before neurogenesis through utilizing its histone H3-K4 demethylation activity Wu and Sun, 2006 .Moreover, it has been shown that heterochromaticmarkers, for instance HP1 proteins, in addition to heterochromatic histone modifications, alter the localization from disseminated and very forceful in ESC to more intense distinctive loci throughout the cellular differentiation. Reduction of multipotency of stem cells results from progressive gene silencing Lunyak and Rosenfeld, 2008 . However, tumor propagation is based on the activity of a subpopulation of CSC which is capable of an atypical type of differentiation that leads to tumor formation, attack, and metastasis to far sites. It is believed that these aberrant precursors powerfully look like regular tissue stem cells, having defining features for instance asymmetric cell division, self-renewal, and the maintenance of an undifferentiated phenotype Polyak and Hahn, 2006 . As normal differentiation is regulated by epigenetic modifications to chromatin, it is almost certain that epigenetics also contributes to the unusual differentiation existing in tumors that include maintenance of multipotency and self-renewal properties of their ancestor cells Muñoz et al., 2012 . Among different models behind cancer propagation, it may assume that CSC could follow more than one pathway which finally depends on genetic or epigenetic changes in the cancer cell. Mainly two theories behind the formation of CSCs are related to epigenetic changes such as CSCs arise from the previously differentiated cell after some modification and epigenetic alterations; the last one is the conversion of immature tissue stem cell or progenitor cell in tumor cell Patra et al., 2010 . Consequently, knowledge of the epigenetic events associated with TSCs behavior will result in a complete understanding of carcinogenesis.
Cancer Stem Cells Surface Molecules
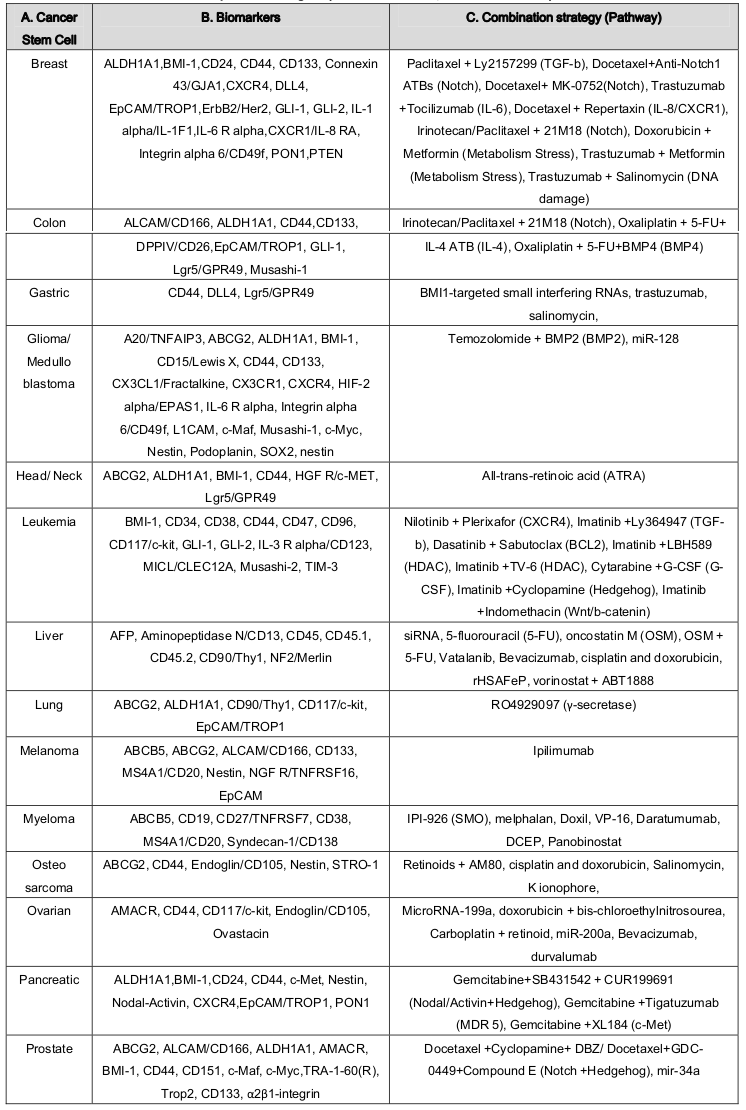
Although some progress has been made in understanding CSCs surface molecules but many issues still remain to be addressed. However, FACs based cell surface markers identification revealed several biomarkers for CSCs identification and isolation Fábián et al., 2012 . Distinct and specific surface biomarker phenotypes can be used to distinguish CSC from other tumor cells and normal stem cells. The LSC displays a CD34 + +CD38 - surface marker phenotype. The loss of CD38 distinguishes LSCs from normal HSCs, although both LSCs and HSCs are CD34 + positive Chen et al., 2013 . Type I CD44 cell-surface ligand is a useful marker for identifying CSCs in breast CSCs Fillmore and Kuperwasser, 2007 . The expression of CD24 is a trademark of an extensive range of epithelial cancers and metastasis which is also used as a marker for CSC. Marker CD133 was identified for breast CSCs but now used for several solid tumors Visvader and Lindeman, 2012 . A marker, PROCR, identified using gene expression profiling of primary BCs, is also a marker of hematopoietic and ESCs Zhang et al., 2014 .In metastasis stage, CXCR4 and ABCG2 could be used as CSC marker in BCs population Bao et al., 2013 . Oxidizing enzyme ALDH1 showed to be increased in CSCs and used to differentiate CSCs from different cancers sample Ricardo et al., 2011 . Dual wavelength FACs with Hoechst 33342 dye efflux can be used for SP cells detection in various human solid malignant tumors including BC Zhang et al., 2014 . Nanog showed distinguishing mark CSCs from normal human neuronal stem cell populations in GSCs Field et al., 2009 . Suetsugu et al., 2010 sorted cancer stem-like and non-stem cells simultaneously in a tumor by real time color-coded imaging Suetsugu et al., 2010 . Table 3 shows cancers stem marker (B) and their distribution (A).
Table 3.
(A) Cancer stem cell types, (B) Identification stem cell marker, and (C) Combined chemotherapy and anti-CSC therapeutic strategies ( Hu and Fu, 2012 Vidal et al., 2013 ).
Targeting Cancer Stem Cell
There are many emerged therapeutic agents against cancers stem cell and are actively being evaluated in pre-clinical models of various cancers as well as in human clinical trials. Vidal et al. 2014 listed current strategies available for suppressing chemotherapy resistance of cancer stem cells by anti-CSC agents in their excellent review article Vidal et al., 2013 . The success of these approaches relies on the identification of molecular pathways, miRNAs and niches that selectively regulate CSC function. Imatinam mediated inhibition of a receptor TK named CD177, has been found to be effective in the treatment of gastrointestinal stromal tumors and chronic myelogenous leukemia Insan and Jaitak, 2014 . Using BCR-ABL–induced CML as a disease model for CSCs showed that BCR-ABL downregulated the Blk gene through c-Myc in LSCs in CML mice and that Blk functions as a tumor suppressor in LSCs but does not affect normal HSCs or hematopoiesis. Blk suppresses LSC function and the proliferation of human CML stem cells through a pathway involving Pax5 and p27 Zhang et al., 2012 . Deonarain et al., 2009 and Pecqueur et al., 2013 reported a therapeutic strategy through antibodies and metabolism Deonarain et al., 2009 Pecqueur et al., 2013 . Lenz and Kahn, 2014 reported that specific CBP ⁄ catenin antagonists appear to have the ability to safely eliminate CSC by taking advantage of an intrinsic differential preference in the way SSC and CSC divide Lenz and Kahn, 2014 . Inhibition of the pathways that promote E-M and CSC plasticity may suppress tumor recurrence following chemotherapy Doherty et al., 2016 .Identification of miRNA as a signature molecule to CSCs and their potential role make them good therapeutic targets for next-generation anti-cancer drugs and directly impact the current efforts in the safe eradication of malignancies. MicroRNA-34a negatively regulated cell proliferation, migration, and invasion and suppresses the breast cancer stem cell-like characteristics by downregulating Notch1 pathway in MCF-7 cells Kang et al., 2015 .MicroRNA mir-34a also has been shown to suppress prostate cancer stem cells and metastasis by directly repressing CD44 Liu et al., 2011 .MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells CD44 (+) /CD117 (+) Cheng et al., 2012 . Inhibition of let-7 results in the increased chemosensitivity of hepatocellular CSCs to sorafenib and doxorubicin, while silencing of miR-181 leads to reduction in HSCs motility and invasion by controlling the aberrant expressions of cytokine IL-6 and transcription factor Twist Meng et al., 2011 .Suppression of oncogenic miRNA-181a in BCSCs epigenetically prevents EMT-related cancer-initiating cell self-renewal Oliveras-Ferraros et al., 2011 .Downregulation of miR-125b-2 expression in GBM derived SCs could allow temozolomide, a chemotherapeutic agent, to induce apoptosis by increasing the cytochrome c release from mitochondria, induction of Apaf-1, activation of caspase-3, poly-ADP-ribose polymerase and proapoptotic protein Bax while decreasing the expression of Bcl-2 Shi et al., 2010 .Specific inhibition of miR-21 by an anti-miR-21 locked nucleic acid increases drug sensitivity of cancer stem cells to anticancer drugs Misawa et al., 2010 .Forced expression of miR-124 and miR-137 in human derived GBM-derived stem cells leads to loss of their self-renewal and oncogenic capacity, leaving normal stem and precursor cells unharmed. Overexpression of miR-128 significantly blocked glioma CSC self-renewal by directly decreasing in histone methylation and Akt phosphorylation, and up-regulation of p2 Li et al., 2009 .In another study, miR145 has been shown to inhibit the tumorigenic and CSC-like abilities by targeting Oct4 and Sox2 in GBM-CD133(+) and facilitated their differentiation into CD133(-)-non-CSCs Yang et al., 2012 . Table 3 summarizes combined chemotherapy and anti-CSC therapeutic strategies (C) for different cancer stem cell types.
Concluding Remarks
The discovery of CSCs in some tumor types has ushered in a new era of cancer research. Therapeutic strategies against multiple malignancies have severe limitations as it is resistance to chemotherapy and radiotherapy. Additionally, they are not precisely specific against CSC and there have the record of recurrence and metastasis because most therapies cannot eliminate CSCs completely. Some therapies targeting at eradicating cancer stem cells which have been developed recent years. Selectively targeting surface markers of cancer stem cells, by intervening crucial signal elements and aberrant pathways, by suppressing specific characteristics of CSCs, in addition, by manipulating tumor microenvironment and using miRNA are the current strategies to fight against cancer. However, to improve the result of treatment of cancer it is needed to characterize not by single antigen markers but through a combination and targeting cancer stem cells without harming their normal counterparts.
References
- N. Atsumi, G. Ishii, M. Kojima, M. Sanada, S. Fujii, A. Ochiai. Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma A431. Biochemical and Biophysical Research Communications. 2008;373:36-41. Google Scholar
- B. Bao, A. Ahmad, A.S. Azmi, S. Ali, F.H. Sarkar. Overview of Cancer Stem Cells (CSCs) and Mechanisms of Their Regulation: Implications for Cancer Therapy. In Current Protocols in Pharmacology (Wiley-Blackwell). 2013;:. Google Scholar
- S.A. Bapat, A. Krishnan, A.D. Ghanate, A.P. Kusumbe, R.S. Kalra. Gene Expression: Protein Interaction Systems Network Modeling Identifies Transformation-Associated Molecules and Pathways in Ovarian Cancer. Cancer Research. 2010;70:4809-4819. Google Scholar
- P.A. Beachy, S.S. Karhadkar, D.M. Berman. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324-331. Google Scholar
- A.F. Chambers, Z. Werb. Invasion and metastasis—recent advances and future challenges. Journal of Molecular Medicine. 2015;93:361-368. Google Scholar
- K. Chen, Y.-h. Huang, J.-l. Chen. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacologica Sinica. 2013;34:732-740. Google Scholar
- Y.-H. Chen, Lucy D.A. McGowan, Patrick J. Cimino, S. Dahiya, Jeffrey R. Leonard, Da Y. Lee, David H. Gutmann. Mouse Low-Grade Gliomas Contain Cancer Stem Cells with Unique Molecular and Functional Properties. Cell Reports. 2015;10:1899-1912. Google Scholar
- W. Cheng, T. Liu, X. Wan, Y. Gao, H. Wang. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS Journal. 2012;279:2047-2059. Google Scholar
- A. Colmone, M. Amorim, A.L. Pontier, S. Wang, E. Jablonski, D.A. Sipkins. Leukemic Cells Create Bone Marrow Niches That Disrupt the Behavior of Normal Hematopoietic Progenitor Cells. Science. 2008;322:1861-1865. Google Scholar
- M. Copland, V. Campbell. Hedgehog signaling in cancer stem cells: a focus on hematological cancers. Stem Cells and Cloning: Advances and Applications. 2015;27:. Google Scholar
- A.A. Dayem, H.-Y. Choi, J.-H. Kim, S.-G. Cho. Role of Oxidative Stress in Stem, Cancer, and Cancer Stem Cells. Cancers. 2010;2:859-884. Google Scholar
- M.P. Deonarain, C.A. Kousparou, A.A. Epenetos. Antibodies targeting cancer stem cells: A new paradigm in immunotherapy?. mAbs. 2009;1:12-25. Google Scholar
- J.T. DeSano, L. Xu. MicroRNA Regulation of Cancer Stem Cells and Therapeutic Implications. The AAPS Journal. 2009;11:682-692. Google Scholar
- M.A. Dickson, E.B. Papadopoulos, C.V. Hedvat, S.C. Jhanwar, R.J. Brentjens. Acute myeloid leukemia arising from a donor derived premalignant hematopoietic clone: A possible mechanism for the origin of leukemia in donor cells. Leukemia Research Reports. 2014;3:38-41. Google Scholar
- M. Doherty, J. Smigiel, D. Junk, M. Jackson. Cancer Stem Cell Plasticity Drives Therapeutic Resistance. Cancers. 2016;8:8. Google Scholar
- S. Eridani. Types of Human Stem Cells and Their Therapeutic Applications. Stem Cell Discovery. 2014;04:13-26. Google Scholar
- Á. Fábián, G. Vereb, J. Szöllősi. The hitchhikers guide to cancer stem cell theory: Markers, pathways and therapy. Cytometry. 2012;83A:62-71. Google Scholar
- M. Field, S. Bushnev, A.A. Alvarez, N. Avgeropoulos, K. Sugaya. Markers Distinguishing Cancer Stem Cells from Normal Human Neuronal Stem Cell Populations in Malignant Glioma Patients. Neurosurgery. 2009;65:426. Google Scholar
- C. Fillmore, C. Kuperwasser. Human breast cancer stem cell markers CD44 and CD24: enriching for cells with functional properties in mice or in man?. Breast Cancer Research. 2007;9:303. Google Scholar
- S. Ghaffari. Cancer, stem cells and cancer stem cells: old ideas, new developments. F1000 Medicine Reports. 2011;3:. Google Scholar
- L. Han, S. Shi, T. Gong, Z. Zhang, X. Sun. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharmaceutica Sinica B. 2013;3:65-75. Google Scholar
- X. He, L. Marchionni, D.E. Hansel, W. Yu, A. Sood, J. Yang, G. Parmigiani, W. Matsui, D.M. Berman. Differentiation of a Highly Tumorigenic Basal Cell Compartment in Urothelial Carcinoma. Stem Cells. 2009;27:1487-1495. Google Scholar
- C.D. House, L. Hernandez, C.M. Annunziata. In vitro Enrichment of Ovarian Cancer Tumor-initiating Cells. Journal of Visualized Experiments. 2015;:. Google Scholar
- Y. Hu, L. Fu. Targeting cancer stem cells: a new therapy to cure cancer patients. Am J Cancer Res. 2012;2:340-356. Google Scholar
- M. Insan, V. Jaitak. New Approaches to Target Cancer Stem Cells: Current Scenario. MRMC. 2014;14:20-34. Google Scholar
- D. Jaworska, W. Król, E. Szliszka. Prostate Cancer Stem Cells: Research Advances. IJMS. 2015;16:27433-27449. Google Scholar
- J. Jiang, C.-c. Hui. Hedgehog Signaling in Development and Cancer. Developmental Cell. 2008;15:801-812. Google Scholar
- L. Kang, J. Mao, Y. Tao, B. Song, W. Ma, Y. Lu, L. Zhao, J. Li, B. Yang, L. Li. MicroRNA-34a suppresses the breast cancer stem cell-like characteristics by downregulating Notch1 pathway. Cancer Science. 2015;106:700-708. Google Scholar
- C. Kantara, M.R. O'Connell, G. Luthra, A. Gajjar, S. Sarkar, R.L. Ullrich, P. Singh. Methods for detecting circulating cancer stem cells (CCSCs) as a novel approach for diagnosis of colon cancer relapse/metastasis. Lab Invest. 2014;95:100-112. Google Scholar
- N.J. Kennedy, C. Cellurale, R.J. Davis. A Radical Role for p38 MAPK in Tumor Initiation. Cancer Cell. 2007;11:101-103. Google Scholar
- L. Lacina, J. Plzak, O. Kodet, P. Szabo, M. Chovanec, B. Dvorankova, K. Smetana Jr. Cancer Microenvironment: What Can We Learn from the Stem Cell Niche. IJMS. 2015;16:24094-24110. Google Scholar
- J.D. Lathia, S.C. Mack, E.E. Mulkearns-Hubert, C.L.L. Valentim, J.N. Rich. Cancer stem cells in glioblastoma. Genes & Development. 2015;29:1203-1217. Google Scholar
- H.-J. Lenz, M. Kahn. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Science. 2014;105:1087-1092. Google Scholar
- L. Li. Normal Stem Cells and Cancer Stem Cells: The Niche Matters. Cancer Research. 2006;66:4553-4557. Google Scholar
- Y. Li, F. Guessous, Y. Zhang, C. DiPierro, B. Kefas, E. Johnson, L. Marcinkiewicz, J. Jiang, Y. Yang, T.D. Schmittgen. MicroRNA-34a Inhibits Glioblastoma Growth by Targeting Multiple Oncogenes. Cancer Research. 2009;69:7569-7576. Google Scholar
- C. Liu, K. Kelnar, B. Liu, X. Chen, T. Calhoun-Davis, H. Li, L. Patrawala, H. Yan, C. Jeter, S. Honorio. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nature Medicine. 2011;17:211-215. Google Scholar
- S. Liu, G. Dontu, M.S. Wicha. . Breast Cancer Research. 2005;7:86. Google Scholar
- M.E.W. Logtenberg, J. Boonstra. Cancer stem cells and addicted cancer cells. Oncol Discov. 2013;1:7. Google Scholar
- V.V. Lunyak, M.G. Rosenfeld. Epigenetic regulation of stem cell fate. Human Molecular Genetics. 2008;17:R28-R36. Google Scholar
- H. Luu, R. Zhang, R. Haydon, E. Rayburn, Q. Kang, W. Si, J. Park, H. Wang, Y. Peng, W. Jiang. Wnt / β-Catenin Signaling Pathway as Novel Cancer Drug Targets. Current Cancer Drug Targets. 2004;4:653-671. Google Scholar
- M. Hongbao, M. Ma.. Stem Cell Introduction. Stem Cell. 2014;5(2):1-10. Google Scholar
- T. Martin. Evaluation of the expression of stem cell markers in human breast cancer reveals a correlation with clinical progression and metastatic disease in ductal carcinoma. Oncology Reports. 2013;:. Google Scholar
- F. Martin-Belmonte, M. Perez-Moreno. Epithelial cell polarity, stem cells and cancer. Nature Reviews Cancer. 2011;:. Google Scholar
- M.J. Mayer, L.H. Klotz, V. Venkateswaran. Metformin and prostate cancer stem cells: a novel therapeutic target. Prostate Cancer and Prostatic Disease. 2015;18:303-309. Google Scholar
- F. Meng, S.S. Glaser, H. Francis, S. DeMorrow, Y. Han, J.D. Passarini, A. Stokes, J.P. Cleary, X. Liu, J. Venter. Functional analysis of microRNAs in human hepatocellular cancer stem cells. Journal of Cellular and Molecular Medicine. 2011;16:160-173. Google Scholar
- B.J. Merrill. Wnt Pathway Regulation of Embryonic Stem Cell Self-Renewal. Cold Spring Harbor Perspectives in Biology. 2012;4:a007971-a007971. Google Scholar
- A. Misawa, R. Katayama, S. Koike, A. Tomida, T. Watanabe, N. Fujita. AP-1-Dependent miR-21 Expression Contributes to Chemoresistance in Cancer Stem Cell-Like SP Cells. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics. 2010;19:23-33. Google Scholar
- S.J. Morrison, J. Kimble. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068-1074. Google Scholar
- P. Muñoz, M.S. Iliou, M. Esteller. Epigenetic alterations involved in cancer stem cell reprogramming. Molecular Oncology. 2012;6:620-636. Google Scholar
- L.V. Nguyen, R. Vanner, P. Dirks, C.J. Eaves. Cancer stem cells: an evolving concept. Nature Reviews Cancer. 2012;:. Google Scholar
- R. Nusse. Wnt signaling and stem cell control. Cell Res. 2008;18:523-527. Google Scholar
- C. Oliveras-Ferraros, S. Cufí, A. Vazquez-Martin, V.Z. Torres-Garcia, S. Del Barco, B. Martin-Castillo, J.A. Menendez. Micro(mi)RNA expression profile of breast cancer epithelial cells treated with the anti-diabetic drug metformin: Induction of the tumor suppressor miRNA let-7a and suppression of the TGFβ-induced oncomiR miRNA-181a. Cell Cycle. 2011;10:1144-1151. Google Scholar
- S.K. Patra, M. Deb, A. Patra. Molecular marks for epigenetic identification of developmental and cancer stem cells. Clinical Epigenetics. 2010;2:27-53. Google Scholar
- D.R. Pattabiraman, R.A. Weinberg. Tackling the cancer stem cells — what challenges do they pose?. Nature Reviews Drug Discovery. 2014;13:497-512. Google Scholar
- C. Pecqueur, L. Oliver, K. Oizel, L. Lalier, F.M. Vallette. Targeting Metabolism to Induce Cell Death in Cancer Cells and Cancer Stem Cells. International Journal of Cell Biology. 2013;2013:1-13. Google Scholar
- L. Persano, E. Rampazzo, G. Basso, G. Viola. Glioblastoma cancer stem cells: Role of the microenvironment and therapeutic targeting. Biochemical Pharmacology. 2013;85:612-622. Google Scholar
- C. Plass, S.M. Pfister, A.M. Lindroth, O. Bogatyrova, R. Claus, P. Lichter. Mutations in regulators of the epigenome and their connections to global chromatin patterns in cancer. Nat Rev Genet. 2013;14:765-780. Google Scholar
- K. Polyak, W.C. Hahn. Roots and stems: stem cells in cancer. Nature Medicine. 2006;12:296-300. Google Scholar
- T. Reya, S.J. Morrison, M.F. Clarke, I.L. Weissman. . Nature. 2001;414:105-111. Google Scholar
- S. Ricardo, A.F. Vieira, R. Gerhard, D. Leitao, R. Pinto, J.F. Cameselle-Teijeiro, F. Milanezi, F. Schmitt, J. Paredes. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. Journal of Clinical Pathology. 2011;64:937-946. Google Scholar
- R. Sancho, C.A. Cremona, A. Behrens. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO reports. 2015;16:571-581. Google Scholar
- S. Sell. On the Stem Cell Origin of Cancer. The American Journal of Pathology. 2010;176:2584-2594. Google Scholar
- A. Sengupta, J.A. Cancelas. Cancer stem cells: A stride towards cancer cure?. J Cell Physiol. 2010;225:7-14. Google Scholar
- M. Shackleton. Normal stem cells and cancer stem cells: similar and different. Seminars in Cancer Biology. 2010;20:85-92. Google Scholar
- J.W. Shay, W.E. Wright. Telomeres and telomerase in normal and cancer stem cells. FEBS Letters. 2010;584:3819-3825. Google Scholar
- L. Shi, J. Zhang, T. Pan, J. Zhou, W. Gong, N. Liu, Z. Fu, Y. You. MiR-125b is critical for the suppression of human U251 glioma stem cell proliferation. Brain Research. 2010;1312:120-126. Google Scholar
- Y. Strulovici, P.L. Leopold, T.P. O'Connor, R.G. Pergolizzi, R.G. Crystal. Human Embryonic Stem Cells and Gene Therapy. Mol Ther. 2007;:. Google Scholar
- A. Suetsugu, Y. Osawa, M. Nagaki, H. Moriwaki, S. Saji, M. Bouvet, R.M. Hoffman. Simultaneous color-coded imaging to distinguish cancer “stem-like” and non-stem cells in the same tumor. Journal of Cellular Biochemistry. 2010;111:1035-1041. Google Scholar
- K. Takahashi, K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda, S. Yamanaka. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861-872. Google Scholar
- D.G. Tang. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457-472. Google Scholar
- M. Topcul, F. Topcul, I. Cetin. . Asian Pacific Journal of Cancer Prevention. 2013;14:2819-2822. Google Scholar
- L. Vermeulen, F. De Sousa E Melo, M. van der Heijden, K. Cameron, J.H. de Jong, T. Borovski, J.B. Tuynman, M. Todaro, C. Merz, H. Rodermond. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biology. 2010;12:468-476. Google Scholar
- S.J. Vidal, V. Rodriguez-Bravo, M. Galsky, C. Cordon-Cardo, J. Domingo-Domenech. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene. 2013;33:4451-4463. Google Scholar
- Jane E. Visvader, Geoffrey J. Lindeman. Cancer Stem Cells: Current Status and Evolving Complexities. Cell Stem Cell. 2012;10:717-728. Google Scholar
- H. Wu, Y.E. Sun. Epigenetic Regulation of Stem Cell Differentiation. Pediatr Res. 2006;59:21R-25R. Google Scholar
- Y.-P. Yang, Y. Chien, G.-Y. Chiou, J.-Y. Cherng, M.-L. Wang, W.-L. Lo, Y.-L. Chang, P.-I. Huang, Y.-W. Chen, Y.-H. Shih. Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials. 2012;33:1462-1476. Google Scholar
- R.L. Yauch, S.E. Gould, S.J. Scales, T. Tang, H. Tian, C.P. Ahn, D. Marshall, L. Fu, T. Januario, D. Kallop. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406-410. Google Scholar
- M.-H. Yoo, D.L. Hatfield. The cancer stem cell theory: is it correct?. Molecules and cells. 2008;26:514. Google Scholar
- Z. Yu, T.G. Pestell, M.P. Lisanti, R.G. Pestell. Cancer stem cells. The International Journal of Biochemistry & Cell Biology. 2012;44:2144-2151. Google Scholar
- H. Zhang, C. Peng, Y. Hu, H. Li, Z. Sheng, Y. Chen, C. Sullivan, J. Cerny, L. Hutchinson, A. Higgins. The Blk pathway functions as a tumor suppressor in chronic myeloid leukemia stem cells. Nature Genetics. 2012;44:861-871. Google Scholar
- M. Zhang, Z. Li, X. Zhang, Y. Chang. Cancer stem cells as a potential therapeutic target in breast cancer. Stem Cell Investigation. 2014;1:. Google Scholar
- B.-B.S. Zhou, H. Zhang, M. Damelin, K.G. Geles, J.C. Grindley, P.B. Dirks. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nature Reviews Drug Discovery. 2009;8:806-823. Google Scholar
- D. Zhou, L. Shao, D.R. Spitz. Reactive Oxygen Species in Normal and Tumor Stem Cells. In Advances in Cancer Research (Elsevier BV). 2014;:1-67. Google Scholar
 Biomedpress
Biomedpress Open Access
Open Access 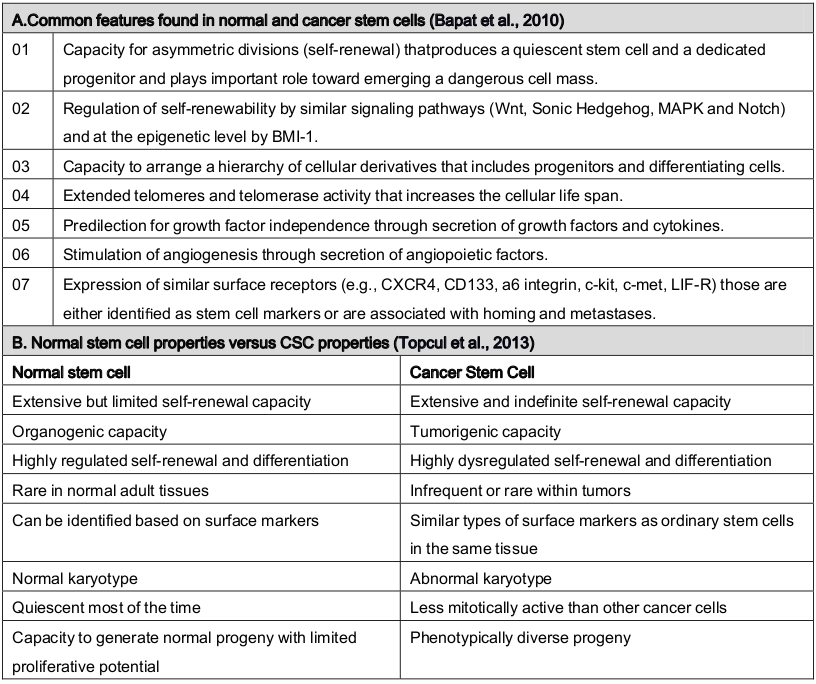







(A) Common characteristics and (B) distinguish between cancer stem cells and stem cells.